Abstract
Tomato product consumption is inversely related to prostate cancer incidence, and lycopene (LYC) has been implicated in reduced prostate cancer risk. The contribution of other tomato carotenoids, phytoene (PE) and phytofluene (PF), towards prostate cancer risk has not been adequately studied. The relative uptake and tissue distribution of tomato carotenoids are not known. We hypothesize that PE and PF are bioavailable from a tomato powder diet or from a purified source and accumulate in androgen-sensitive tissues. In this study, 4 wk old male Fisher 344 rats were pre-fed an AIN-93G powder diet composed of 10% tomato powder containing PE, PF, and LYC (0.015, 0.012, and 0.011 g/kg diet, respectively). After 30 d tomato powder feeding, hepatic PF concentrations (168 ± 20 nmol/g) were higher than PE or LYC (104 ± 13 and 104 ± 13 nmol/g, respectively). In contrast, LYC, followed by PF, had the highest accumulation of the measured carotenoids in the prostate lobes and seminal vesicles. When tomato powder-fed rats received a single oral dose of either ∼2.7 mg PE or PF, an increase in the dosed carotenoid concentration was observed in all measured tissues, except the adrenal. Percent increases of PF were greater than that of PE in liver, serum, and adipose (37, 287 and 49% versus 16, 179 and 23%, respectively). Results indicate that the relative tomato carotenoid biodistribution differs in liver and androgen-sensitive tissues, suggesting that minor changes in the number of sequential double bonds in carotenoid structures alter absorption and/or metabolism of tomato carotenoids.
Keywords: Carotenoids, Phytoene, Phytofluene, Lycopene, Prostate Cancer, Rats
1. Introduction
Prostate cancer is the third leading cause of cancer death in American men [1]. In the U.S., over 230,000 new prostate cancer cases were diagnosed and over 27,000 deaths occurred in 2006 [1]. Epidemiological evidence strongly indicates that a diet rich in fruits and vegetables is associated with a reduced risk of cancer [2], therefore approaches for prostate cancer prevention that focus on both nutrition and chemopreventive strategies are critically needed [3]. With respect to tomatoes, epidemiological data suggest a relationship between higher intake of fresh and processed tomato products and reduced risk of several types of cancer [4]. More specifically, increased tomato consumption has been significantly associated with reduced risk of prostate cancer in several epidemiological studies, and thus investigating the protective health benefits of tomatoes is an active area of prostate cancer research [5-8].
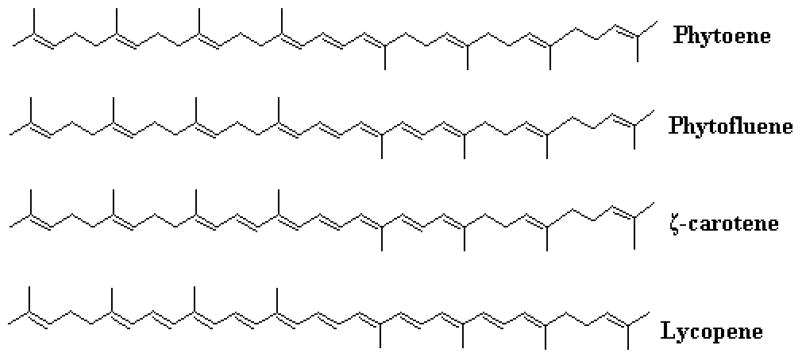
Carotenoids are natural yellow, orange, and red pigments found in fruits and vegetables, and have a wide range of proposed biological functions including antioxidant and anticarcinogen properties, and immunoprotection [8]. Lycopene (LYC) has recently been the primary focus of both in vitro and in vivo studies examining the relationship between increased intake of tomatoes and reduced risk of prostate cancer. While LYC is the major carotenoid in tomatoes, other carotenoid precursors of LYC, including phytoene (PE) and phytofluene (PF) are also present in substantial amounts [9], yet few research studies have been conducted with these carotenoids. Polyene structures of tomato carotenoids are depicted in Figure 1. Although the concentrations of PE and PF in tomatoes are usually lower than that of LYC, one small study suggests that significant amounts of both PE and PF have been detected in human serum and tissues, including liver, lung, breast, colon, skin, and prostate [10].
Figure 1.
Chemical structures of carotenoids found in tomatoes. Lycopene is the predominant tomato carotenoid, but significant amounts of other carotenoids including phytoene and phytofluene are present as well.
Our laboratory has demonstrated in an N-methyl-N-nitrosurea (MNU)-androgen induced rat carcinogenesis model and in a Dunning R-3327H transplantable prostate adenocarcinoma model, that diets containing 10% whole tomato powder significantly inhibited the development of prostate cancer as compared to a control diet, whereas a LYC-supplemented diet was not as effective [11,12]. Short-term clinical studies with prostate cancer patients have reported therapeutic benefits with tomato supplementation, including reductions in prostate size, leukocyte DNA damage, and prostate specific antigen levels, as well as positively modulated volume and grade of prostate intraepithelial neoplasia, and also altered biomarkers of prostate cell growth and differentiation [13,14]. Collectively, results from these studies strongly suggest that tomato products contain phytochemicals, in addition to LYC, that may modify prostate carcinogenesis. Due to the substantial amounts of PE and PF in tomatoes and the presence of these carotenoids in human tissues and serum, it is essential to elucidate their potential health benefits.
The relative bioavailability of PE or PF, as well as tissue biodistributions of tomato carotenoids after consumption of a tomato powder-containing diet has not been adequately determined in an appropriate animal model. It is imperative to elucidate if PE and PF, as compared to LYC, are bioavailable and accumulate in tissues under physiological conditions, before determining their possible preventative roles in prostate carcinogenesis. To improve our understanding of tomato carotenoids and prostate health, we utilized a prostate specific animal model, the Fisher 344 (F344) rat, to first assess tissue and serum biodistributions of tomato carotenoids, including PE and PF, after 30 d consumption of a 10% tomato powder diet. Secondly to determine the relative bioavailability of a single oral dose of purified PE or PF after pre-feeding a 10% tomato powder diet for 30 d. To date very little is known about the differential uptake of PE and PF in their proper isomeric forms, and thus this study provides new insight into the differential accumulation of the three most prominent tomato carotenoids in both androgen and non-androgen sensitive tissues, as well as the bioavailability of PE and PF. By using an animal model that has a prostate homologous to that of humans, these results should be translated to what may occur in the human prostate.
2. Methods and Materials
2.1. Chemicals
PE, PF, and LYC standards (crystalline form) were gifts from BASF (Ludwigshafen, Germany). ζ-Carotene (ZC) standards (crystalline form) were purchased from CaroteNature (Lupsingen, Switzerland). PE and PF were dissolved in petroleum ether, whereas ZC and LYC were dissolved in hexane. Absorbance of all standards was determined spectrophotometrically (phytoene λmax = 286 nm, E1%1cm = 1250; phytofluene λmax = 348 nm, E1%1cm = 1350; ζ-carotene λmax = 400 nm, E1%1cm = 2555; lycopene λmax = 472 nm, E1%1cm = 3450). HPLC-photodiode array (PDA) analyses determined the purity of all standards to be ≥98%. All other chemicals, unless otherwise noted, were purchased from Fisher Scientific (Fairlawn, NJ).
2.2. Animals and experimental design
The study was approved by the University of Illinois Laboratory Animal Care and Use Committee and followed all necessary protocols to ensure the humane treatment of the animals. Male F344 rats (21 d of age; n=24) were purchased from Harlan (Indianapolis, IN) and were acclimated to their new environment for one week. Throughout the study, the rats were kept under conditions of uniform humidity and temperature on a 12-hour light-dark cycle and individually housed in hanging wire bottom cages to reduce coprophagy, and were weighed every other day. At 28 d of age, all rats were fed powdered AIN-93G diet, containing 10% tomato powder for 30 d (diet described below in section 2.3). To prevent carotenoid degradation, fresh 10% tomato powder diet was provided every other day to all animals. At 48 d of age, tomato powder-fed rats were randomly assigned to 3 treatment groups (n =8), and treatment groups were as follows: 1) cottonseed oil only control-dosed rats; 2) PE-dosed rats; and 3) PF-dosed rats. Based on these treatment groups, at 58 d of age, non-fasted rats were orally intubated with either a control (cottonseed oil) or single dose of PE (2.7 mg) or PF (2.7 mg) provided in 0.5 mL cottonseed oil (carotenoid doses described below in section 2.3). The tomato powder-fed control rats were euthanized approximately 4 h after receiving the control cottonseed oil dose, whereas the PE- and PF-dosed rats were euthanized approximately 24 h after receiving carotenoid doses. Rats were anesthetized with CO2, and blood was taken via cardiac puncture. Rats were subsequently euthanized by CO2 asphyxiation, and the liver, adrenal, adipose, spleen, lung, testes, and prostate-seminal vesicle complex were collected. The prostate-seminal vesicle complex was dissected on ice into four lobes: seminal vesicles, dorsolateral lobe, anterior lobe, and ventral lobe. The anterior and dorsolateral prostate lobes were pooled for analysis. Due to the small tissue amount obtained from each rat, it was necessary to pool all prostate tissue samples within each group to obtain accurate analysis of carotenoid accumulation. All harvested tissues were weighed, immersed in liquid nitrogen, and subsequently stored at −80 °C.
2.3. Diet and carotenoid doses
All rats consumed a powdered AIN-93G semi purified diet enriched with 10% freeze-dried, whole tomato powder (0.015 g PE/kg, 0.012 g PF/kg, 0.011 g LYC/kg, and 0.001 g ZC/kg diet; Gilroy Foods, Gilroy, CA) ad libitum for 30 d. While it would be expected that LYC would be the predominant carotenoid in the tomato powder, these relative carotenoid concentrations have been found in other lots of this tomato powder from this company. Rats fed the 10% tomato powder diet consumed approximately 0.21 mg PE/d, 0.17 mg PF/d, 0.15 mg LYC/d, and 0.01 mg ZC/d. When adding the freeze-dried, whole tomato powder to the diet, the macronutrients and micronutrients were adjusted to equally match that of the typical AIN-93G diet. The composition of macronutrients and of the mineral and vitamin mixes has been described [15] and were used previously [11]. The diet was stored in the dark at 4 °C.
Purified, analytical standards of PE and PF as various cis-isomers (BASF) were used for the oral carotenoid doses. On the day of dosing, new standard vials were opened. PE and PF were individually reconstituted in chloroform and added to 6.0 mL of cottonseed oil. Chloroform was evaporated before dosing to make final concentrations of 5.4 mg PE/mL and 5.4 mg PF/mL. The total amount of PE or PF dosed per rat was 2.7 ± 0.1 mg. Carotenoid solubility in oil was ensured by observations under a light microscope. Carotenoids are susceptible to isomerization and oxidation, therefore precautions were taken including preparation of the carotenoid doses under yellow lights, keeping carotenoid-chloroform solutions on ice before addition to oil, and purging the oil doses with argon to remove chloroform.
2.4. Tissue and serum carotenoid extraction and quantification
Tissue and serum extraction and analysis was performed as previously described [16]. Briefly, tissue or serum samples were combined with a KOH/ethanol solution (1:5) containing 0.1% BHT. Tissues were saponified at 60 °C for 30 min (serum was not saponified). Samples were then placed on ice, and deionized water was added. Tissue and serum carotenoids were extracted four times with addition of hexane. Hexane extracts were dried in a Speedvac concentrator (model AS160; Savant, Farmingdale, NY), flushed with argon, and stored at −20 °C for ≤ 24 h before HPLC-PDA analysis. All carotenoid extracts were kept on ice and under yellow lights throughout the extraction process.
Carotenoid concentrations in tissue and serum samples were quantified by a previously described HPLC-PDA system [16-18], and the HPLC mobile phases and gradient procedure utilized in this study have been previously described [19]. In brief, samples from each carotenoid-enriched tissue extract were dissolved in 32-40 µL methyl-tert-butyl ether (MTBE) and injected onto a C30, 4.6 × 150 mm analytical column (YMC, Wilmington, NC, USA) maintained at 25 °C. Qualitative and quantitative analysis was conducted with a HPLC-PDA system consisting of a Waters 991 detector (Millipore, Milford, MA, USA) monitored at 200-600 nm, a Rainin Dynamics gradient pump system model SD-200 (Walnut Creek, CA, USA), and a Varian Prostar pump model 210 (Woburn, NC, USA). Carotenoid isomers were qualitatively identified through comparison to UV spectra and retention times of analytical standards. Serum and tissue carotenoid concentrations were quantified for total PE, total PF, total LYC and total ZC isomer concentrations.
2.5. Statistical analysis
A complete randomized design was used to assign rats to the different treatment groups. Differences among treatment groups were analyzed by one-way ANOVA, and group mean comparisons were further analyzed by the post-hoc Tukey's studentized range test with α=0.05 and 0.01 [20]. This statistical analysis has been previously utilized in prior studies [16,18]. All statistical analyses were conducted with SAS (version 8.1; SAS Institute, Cary, NC, USA). The prostate and seminal vesicle tissues were pooled within groups, resulting in one data point, thus statistical analysis was not performed for these tissues. Results were expressed as means ± SEM, unless otherwise indicated.
3. Results
3.1. Food intake and weight gain
Food intake and weight gain did not differ among treatment groups throughout the study. No adverse effects of diet or administered dose were observed.
3.2. Tissue biodistribution of tomato carotenoids in F344 rats pre-fed 10% tomato powder diet
HPLC analyses of the serum and tissues from the tomato powder-fed rats illustrated that there was a differential accumulation and distribution of tomato carotenoids in serum, non-androgen and androgen sensitive tissues. Of the total carotenoid composition in the experimental diet, ∼40%, 30%, 28%, and 2% consisted of PE, PF, LYC and ZC, respectively. Among the carotenoids, PE concentration in the serum was greatest, while PF, LYC, and ZC were found at lower levels in the serum of tomato powder-fed rats (Table 1). In contrast, PF had the greatest accumulation in the liver, whereas PE, LYC, and ZC were at lower concentrations. In the adrenal, PE and PF concentrations were approximately equal and far greater than that of LYC or ZC. LYC, in contrast, accumulated to the greatest extent in the spleen and adipose.
Table 1.
Carotenoid concentrations in serum and non-androgen sensitive tissues of rats fed a 10% tomato powder diet for 30 d and supplemented with a single oral dose of PE or PF on d 31.1
| Carotenoid | Tomato powder-fed 2 | PE-24hr 3 | PF-24hr 4 |
|---|---|---|---|
| Serum (nmol/L) | |||
| Phytoene | 556 ± 55 c | 1550 ± 240 d | 508 ± 32 c |
| Phytofluene | 403 ± 33c | 451 ± 34 c | 1560 ± 160 d |
| Lycopene | 288 ± 19 | 342 ± 40 | 313 ± 28 |
| ζ-Carotene | 48 ± 3 | 51 ± 4 | 48 ± 3 |
| Liver (nmol/g) | |||
| Phytoene | 104.4 ± 13.3 a,b | 121.6 ± 4.8 b | 82.1 ± 5.9 a |
| Phytofluene | 168.2 ± 20.7 a | 119.5 ± 5.2 a | 230.0 ± 15.1 b |
| Lycopene | 103.6 ± 13.7 | 70.5 ± 6.5 | 82.8 ± 9.3 |
| ζ-Carotene | 15.4 ± 1.9 | 11.2 ± 0.6 | 12.2 ± 0.8 |
| Adrenal (nmol/g) | |||
| Phytoene | 36. 8± 1.6 c | 24.8 ± 1.7 d | 26.7 ± 1.6 c, d |
| Phytofluene | 39.6 ± 1.6 c | 28.4 ± 1.9 d | 34.8 ± 1.7 c, d |
| Lycopene | 7.7 ± 0.9 | 5.8 ± 0.3 | 6.4 ± 0.6 |
| ζ-Carotene | 5.5 ± 0.3 | 4.1 ± 0.4 | 4.5 ± 0.3 |
| Spleen (nmol/g) | |||
| Phytoene | 9.5 ± 0.4 c | 21.8 ± 2.3 d | 9.8 ± 0.3 c |
| Phytofluene | 41.1 ± 1.3 c | 41.8 ± 1.6 c | 61.5 ± 2.1 d |
| Lycopene | 60.2 ± 3.6 | 60.1 ± 2.7 | 56.7 ± 2.2 |
| ζ-Carotene | 4.4 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.1 |
| Adipose (nmol/g) | |||
| Phytoene | 0.15 ± 0.02 a,b | 0.19 ± 0.03 b | 0.11 ± 0.01 a |
| Phytofluene | 0.19 ± 0.02 a | 0.16 ± 0.01 a | 0.28 ± 0.02 b |
| Lycopene | 0.21 ± 0.03 | 0.18 ± 0.01 | 0.22 ± 0.01 |
| ζ-Carotene | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.08 ± 0.00 |
Values are means ± SEM; liver, spleen, serum, and adipose, n = 8; adrenal, n = 3;
Tomato powder-fed = rats pre-fed 10% tomato powder diet for 30 d
PE-24hr = rats, pre-fed 10% tomato powder diet for 30 d, orally gavaged with 2.7 mg PE and euthanized 24 hr post-dose
PF-24hr = rats, pre-fed 10% tomato powder diet for 30 d, orally gavaged with 2.7 mg PF and euthanized 24 hr post-dose
Means within a row with a different letter are statistically different (P < 0.05) determined by one-way ANOVA and post-hoc Tukey's studentized range test.
Means within a row with a different letter are statistically different (P < 0.01) determined by one-way ANOVA and post-hoc Tukey's studentized range test.
Of the measured carotenoids, LYC had the greatest accumulation in androgen sensitive tissues, including the seminal vesicles, ventral, dorsolateral and anterior prostate lobes (Table 2). In fact, LYC prostate lobe concentrations were approximately two-fold greater than PF and approximately four-fold greater than PE and ZC. In contrast, the testes accumulated approximately the same quantities of both PF and LYC in the tomato powder-fed rats.
Table 2.
Carotenoid concentrations in androgen sensitive tissues of rats fed a 10% tomato powder diet for 30 d and supplemented with a single oral dose of PE or PF on d 31.1
| Carotenoid | Tomato powder-fed 2 | PE-24hr 3 | PF-24hr 4 |
|---|---|---|---|
| Testes (nmol/g) | |||
| Phytoene | 0.10 ± 0.02 a | 0.16 ± 0.01 b | 0.08 ± 0.01 a |
| Phytofluene | 0.18 ± 0.01 a | 0.16 ± 0.01 a | 0.23 ± 0.01 b |
| Lycopene | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.01 |
| ζ-Carotene | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 |
| Dorsolateral plus anterior prostate (nmol/g) | |||
| Phytoene | 0.05 | 0.08 | 0.04 |
| Phytofluene | 0.08 | 0.07 | 0.14 |
| Lycopene | 0.22 | 0.22 | 0.21 |
| ζ-Carotene | 0.05 | 0.04 | 0.04 |
| Ventral prostate (nmol/g) | |||
| Phytoene | 0.05 | 0.10 | 0.06 |
| Phytofluene | 0.11 | 0.11 | 0.16 |
| Lycopene | 0.22 | 0.25 | 0.22 |
| ζ-Carotene | 0.05 | 0.06 | 0.05 |
| Seminal vesicles (nmol/g) | |||
| Phytoene | 0.06 | 0.07 | 0.06 |
| Phytofluene | 0.06 | 0.05 | 0.10 |
| Lycopene | 0.10 | 0.10 | 0.11 |
| ζ-Carotene | 0.04 | 0.03 | 0.03 |
Values are means ± SEM; testes n = 8; ventral prostate, dorsolateral plus anterior prostate, and seminal vesicles, n = 1.
Tomato powder-fed = rats pre-fed 10% tomato powder diet for 30 d
PE-24hr = rats, pre-fed 10% tomato powder diet for 30 d, orally gavaged with 2.7 mg PE and euthanized 24 hr post-dose
PF-24hr = rats, pre-fed 10% tomato powder diet for 30 d, orally gavaged with 2.7 mg PF and euthanized 24 hr post-dose
Means within a row with a different letter are statistically different (P < 0.05) determined by one-way ANOVA and post-hoc Tukey's studentized range test.
3.3. Accumulation of PE or PF in various tissues and serum following a single oral carotenoid dose
When rats were pre-fed a 10% tomato diet received a single oral dose of either ∼2.7 mg PE or PF, an increase in content of that carotenoid was observed in all tissues, except the adrenal glands (Tables 1 and 2). Rats orally gavaged with PE had significant increases in PE concentrations in the serum (P < 0.01), spleen (P < 0.01), adipose (P < 0.05), and testes (P < 0.05) after 24 hr, but there was no significant increase of PE in liver. Although no statistical evaluation could be made, prostate and seminal vesicle PE concentrations were increased after 24 hr. Rats orally gavaged with PF also had significant increases in PF concentrations in the serum (P < 0.01), liver (P < 0.05), spleen (P < 0.01), adipose (P < 0.05), and testes (P < 0.05) after 24 hr. Again, although no statistical evaluation could be made, increases in PF concentrations were evident in the seminal vesicles and prostate lobes after 24 hr. With oral provision of either PE or PF, there was no significant reduction of either LYC or ZC in the measured tissues and serum. The percent increases of PE and PF in the liver, serum, and adipose were calculated from data of Table 1. The percent increases of PF in the liver, serum, and adipose were 37%, 287%, and 49%, respectively, and these values were greater than that of PE (16%, 179%, and 23%, respectively).
4. Discussion
Due to the substantial quantities of PE and PF in tomatoes and research studies supporting the relationship between increased tomato consumption and reduced prostate cancer risk, it is essential to begin evaluating the potential biological effects of PE and PF, in addition to LYC. The first goal of the current study was designed to determine biodistribution of tomato carotenoids, including PE, PF, ZC, and LYC, in F344 rats after 30 d consumption of a 10% tomato powder diet in androgen sensitive and non-androgen sensitive tissues and serum. The second objective of this study was to determine the relative bioavailability of PE and PF after provision of a single purified oral dose of either carotenoid. Rats that received PE or PF were pre-fed a 10% tomato powder diet in an attempt to create a tomato-fed state comparable to that of typical Western men. The F344 rat model was used in this study as our laboratory has previously utilized the F344 rat to evaluate 10% tomato powder or LYC consumption and prostate cancer risk [11,18,21,22]. Moreover, the dorsolateral lobe of the F344 rat prostate is similar to that of humans, both histological and in terms of hormonal responsiveness [23]. Although the gerbil and ferret models absorb carotenoids more similar to that of humans, these animals do not have a prostate, and therefore determining the uptake of these compounds into the prostate would not have been possible. As there are slight differences between how rodents and humans absorb carotenoids, this could be viewed as a potential limitation of this study.
Several novel observations concerning the tissue accumulation of tomato carotenoids were revealed in this experiment. Rats fed a 10% tomato powder diet, containing PE, PF, LYC, and ZC, for 30 d exhibited a tissue specific accumulation of tomato carotenoids. The serum profile of carotenoids, with PE having the greatest serum concentration, was expectedly consistent with the relative daily intakes of carotenoids, and therefore reflects recent dietary carotenoid absorption. However, this relative dietary proportion of carotenoid concentrations was not found in the animal tissues. Specifically, the tomato-powder fed rats had approximately 60% greater liver PF concentrations than that of LYC or PE. In contrast, LYC had the greatest carotenoid accumulation in the androgen-sensitive tissues, including the seminal vesicles, dorsolateral, anterior, and ventral prostate lobes, as well as in the spleen. PE and PF had surprisingly high concentrations in the adrenal, as compared to LYC.
When rats were provided with an oral dose of either PE or PF, mean PE and PF concentrations increased in the liver, serum, spleen, adipose, testes, prostate lobes, and seminal vesicles 24 h after carotenoid supplementation. Interestingly, the percent increases of PF in the liver, serum, and adipose were greater than the percent increases of PE. Additionally, when comparing data between the PE and PF gavaged rats, there were numerically greater tissue and serum accumulations of PF than that of PE, suggesting enhanced relative bioavailability of PF. To our knowledge, this study is the first to systematically evaluate the uptake and bioavailability of an oral dose of purified PE or PF in an animal model. Another previous study evaluating the PE and PF bioavailability reported that Sprague-Dawley rats fed a PE- and PF-containing algal diet for two weeks accumulated PE and PF in the plasma, liver, adrenal, kidney, and spleen, but did not report prostate data [24].
Results from the current study depicting a tissue specific accumulation of tomato carotenoids in serum, non-androgen, and androgen sensitive tissues are intriguing. As previously reported, PE and PF accumulate in the liver of F344 rats fed a tomato lipid extract diet for 10 wk [25]. In that study, Zhao and colleagues reported that the PE and PF uptake by the liver was much higher than expected, based on the relatively low percentage of these compounds in the tomato extract [25]. In healthy human subjects supplemented with tomato juice for 4 wk, mean changes from baseline plasma concentrations of PE and PF were greater than that of LYC, despite lower concentrations of PE and PF in tomato juice [26]. In the current study, rats fed a 10% tomato powder diet, containing similar PE, PF, and LYC concentration, accumulated more PE and PF in the liver and serum than LYC. Collectively, results presented in this study and others [25,26] confirm that the apparent bioavailability of PE and PF is at least equivalent, and perhaps better, than that of LYC.
In this experiment, prostate carotenoid concentrations were lower than carotenoid concentrations in the other measured tissues. LYC was the predominant carotenoid in the seminal vesicles, ventral, dorsolateral, and anterior prostate lobes when rats were fed tomato powder, yet measurable quantities of PE, PF, and ζ-carotene were also present. Recently, we have also shown that 14C-phytoene accumulates in human DU 145 prostate cancer cells after a 2 d incubation period [17]. In human prostate, LYC is the most abundant carotenoid [10,27], but a variety of other tomato carotenoids also accumulate [10,14]. The prostate carotenoid concentration profile reported in this animal study is quite similar to that found in human prostate tissue (LYC > PF > PE) [10], further substantiating the use of the F344 rat model for evaluation of tomato carotenoid metabolism, bioavailability, and biodistribution. Because the prostate samples had to be pooled within groups for analysis, we do not know what the variability would be from animal to animal and this necessity could be viewed as a potential limitation of this study.
Due to similar carotenoid concentrations and structures, we had anticipated equivalent tissue uptake and accumulation of PE, PF, and LYC in the tomato powder-fed rats. In light of the present work, it is plausible that there is selective tissue uptake and/or degradation of specific carotenoids. For differential uptake, the observed tissue specific carotenoid biodistribution would suggest the presence of selective binding or transport proteins. Lutein, zeaxanthin, and β-carotene reportedly utilize a transport and/or binding protein for specific tissue uptake [28-33], yet transport proteins for PE, PF, or LYC have not been identified. In CaCo-2 cells, differential and competitive uptake of α-carotene, β-carotene, lutein, and LYC has been reported [34]. Recently, scavenger receptor class B, type I (SR-BI) has been identified as a protein involved in intestinal transport of carotenoids in Caco-2 cells [35], and SR-BI is highly concentrated in human and rat liver and in steroidogenic tissues [36,37]. The specificity of transport and/or binding proteins is unclear, yet it is plausible that this specificity may depend on the number and/or location of conjugated double bonds present within carotenoids (Figure 1).
Differential tissue degradation of carotenoids may also contribute to alterations in carotenoid biodistributions. For example, we speculate that the liver accumulated less LYC than PF due to both enzymatic and non-enzymatic degradation of LYC. The liver is a highly metabolic tissue, and tissue-specific cytochrome P450s produce significant quantities of reactive oxygen species (ROS). LYC is the most effective singlet oxygen quencher in vitro of all C40 carotenoids [38], and by serving as an antioxidant, LYC might be preferentially non-enzymatically degraded in the liver.
Furthermore, liver degradation of LYC may be due to enzymatic cleavage of LYC to apo-carotenals. In vitro studies suggest that LYC is primarily cleaved eccentrically at the 9′,10′ double bond by carotenoid-9′,10′ monooxygenase II (CMO II) enzyme [39], yet LYC may also be centrally cleaved by carotenoid-15, 15′ monooxygenase (CMO I) [40]. CMO I is the key enzyme involved in the metabolism of provitamin A carotenoids, such as β-carotene, to retinal for subsequent conversion to retinol and retinoic acid [41]. Both CMO I and CMO II are highly expressed in mouse and human liver [41,42]. Indeed, LYC metabolites, including apo-8′-lycopenal and apo-10′-lycopenal, are in the liver of LYC-fed rats and ferrets, respectively, suggesting LYC cleavage by CMO II [43,44]. Therefore, if PE and PF have less affinity for CMO I and II, then enzymatic and non-enzymatic cleavage of LYC in the liver would ultimately result in decreased hepatic LYC concentrations relative to PE and PF. In contrast, androgen-sensitive tissues, including the prostate lobes and seminal vesicles, had higher relative amounts of LYC than other measured carotenoids. CMO I and II are expressed in the rodent prostate, but at much lower levels than that of the liver [42,45]. This suggests that enzymatic degradation of LYC in the prostate may be lower than LYC degradation in the liver. In addition, if one assumes that the metabolic activity of the prostate is less than that of the liver, non-enzymatic degradation of LYC in the prostate would be less.
In conclusion, this experiment is the first study to provide new insight into differential tomato carotenoid biodistributions in a rat model pre-fed tomato powder. Specifically, PF and LYC have greater relative carotenoid accumulation in liver and prostate, respectively. Data also indicate significant relative increases of PE or PF in tissues and serum 24 hr after a single oral dose of PE or PF. By using an animal model that has a prostate homologous to that of humans, these novel results may be translatable to conditions in the human prostate. Therefore, continued mechanistic evaluation of PE and PF in prostate cancer prevention, as compared to LYC, is warranted due to these detectable PE and PF prostate concentrations. Future studies should focus on the in vivo metabolism of PE and PF in a similar manner to studies that have been accomplished for LYC metabolism [16,46]. As 14C- PE and 14C- PF sources are not available, biosynthesis of these and other carotenoids through in vitro plant cell culture methodologies is necessary [17]. Precise qualitative and quantitative identification of 14C- carotenoid metabolites throughout the body, with an emphasis on the prostate [16,46], are essential to evaluate potential mechanisms of actions by which tomato carotenoids may act in prostate cancer prevention. Results from this work provide a better understanding of relative PE and PF tissue accumulation, compared to LYC (information not previously known).
Acknowledgments
This work was funded in part by the Initiative for Future Agriculture and Food Systems/U.S. Department of Agriculture 00-52101-9695 and National Institutes of Health/National Cancer Institute CA 112649-01A1. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the U.S. Department of Agriculture or National Institutes of Health/National Cancer Institute. We thank Hansgeorg Ernst of BASF (Ludwigshafen, Germany) for the generous gifts of phytoene and phytofluene.
Abbreviations
- CMO I
carotenoid-15, 15′ monooxygenase
- CMO II
carotenoid-9′,10′ monooxygenase II
- LYC
lycopene
- PE
phytoene
- PF
phytofluene
- ROS
reactive oxygen species
- RP-HPLC-PDA
reverse phase-high pressure liquid chromatography-photodiode array
- ZC
ζ-carotene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer Facts & Figures. American Cancer Society; 2006. [May 1, 2006]. [Google Scholar]
- 2.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer. 1992;18(1):1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 3.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349(4):366–81. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91(4):317–31. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 5.Miller EC, Giovannucci E, Erdman JW, Jr, Bahnson R, Schwartz SJ, Clinton SK. Tomato products, lycopene, and prostate cancer risk. Urol Clin North Am. 2002;29(1):83–93. doi: 10.1016/s0094-0143(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87(23):1767–76. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94(5):391–8. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 8.Campbell JK, Canene-Adams K, Lindshield BL, Boileau TW, Clinton SK, Erdman JW., Jr Tomato phytochemicals and prostate cancer risk. J Nutr. 2004;134 12:3486S–92S. doi: 10.1093/jn/134.12.3486S. [DOI] [PubMed] [Google Scholar]
- 9.Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW., Jr The tomato as a functional food. J Nutr. 2005;135(5):1226–30. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 10.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med. 2002;227(10):845–51. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- 11.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95(21):1578–86. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 12.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67(2):836–43. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, van Breemen R, Ashton D, Bowen PE. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93(24):1872–9. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- 14.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, Li YW, Banerjee M, Grignon D, Bertram JS, Crissman JD, Pontes EJ, Wood DP., Jr Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10(8):861–8. [PubMed] [Google Scholar]
- 15.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 16.Zaripheh S, Boileau TW, Lila MA, Erdman JW., Jr [14C]-lycopene and [14C]-labeled polar products are differentially distributed in tissues of F344 rats prefed lycopene. J Nutr. 2003;133(12):4189–95. doi: 10.1093/jn/133.12.4189. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JK, Rogers RB, Lila MA, Erdman JW., Jr Biosynthesis of 14C-phytoene from tomato cell suspension cultures (Lycopersicon esculentum) for utilization in prostate cancer cell culture studies. J Agric Food Chem. 2006;54(3):747–55. doi: 10.1021/jf0581269. [DOI] [PubMed] [Google Scholar]
- 18.Boileau TW, Clinton SK, Zaripheh S, Monaco MH, Donovan SM, Erdman JW., Jr Testosterone and food restriction modulate hepatic lycopene isomer concentrations in male F344 rats. J Nutr. 2001;131(6):1746–52. doi: 10.1093/jn/131.6.1746. [DOI] [PubMed] [Google Scholar]
- 19.Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64(4):594–602. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates; 1983. [Google Scholar]
- 21.Boileau TW, Boileau AC, Erdman JW., Jr Bioavailability of all-trans and cis-isomers of lycopene. Exp Biol Med. 2002;227(10):914–9. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 22.Boileau TW, Clinton SK, Erdman JW., Jr Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male F344 rats. J Nutr. 2000;130(6):1613–8. doi: 10.1093/jn/130.6.1613. [DOI] [PubMed] [Google Scholar]
- 23.Bosland MC. Use of animal models in defining efficacy of chemoprevention agents against prostate cancer. Eur Urol. 1999;35(56):459–63. doi: 10.1159/000019879. [DOI] [PubMed] [Google Scholar]
- 24.Werman MJ, Mokady S, Ben-Amotz A. Bioavailability of the isomer mixture of phytoene and phytofluene-rich alga Dunaliella bardawil in rat plasma and tissues. J Nutr Biochem. 2002;13(10):585–91. doi: 10.1016/s0955-2863(02)00210-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Khachik F, Richie JP, Jr, Cohen LA. Lycopene uptake and tissue disposition in male and female rats. Proc Soc Exp Biol Med. 1998;218(2):109–14. doi: 10.3181/00379727-218-44283a. [DOI] [PubMed] [Google Scholar]
- 26.Paetau I, Khachik F, Brown ED, Beecher GR, Kramer TR, Chittams J, Clevidence BA. Chronic ingestion of lycopene-rich tomato juice or lycopene supplements significantly increases plasma concentrations of lycopene and related tomato carotenoids in humans. Am J Clin Nutr. 1998;68(6):1187–95. doi: 10.1093/ajcn/68.6.1187. [DOI] [PubMed] [Google Scholar]
- 27.Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW., Jr cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5(10):823–33. [PubMed] [Google Scholar]
- 28.Amano O, Kanda T, Ono T, Iseki S. Immunocytochemical localization of rat intestinal 15 kDa protein, a member of cytoplasmic fatty acid-binding proteins. Anat Rec. 1992;234(2):215–22. doi: 10.1002/ar.1092340208. [DOI] [PubMed] [Google Scholar]
- 29.Tabunoki H, Sugiyama H, Tanaka Y, Fujii H, Banno Y, Jouni ZE, Kobayashi M, Sato R, Maekawa H, Tsuchida K. Isolation, characterization, and cDNA sequence of a carotenoid binding protein from the silk gland of Bombyx mori larvae. J Biol Chem. 2002;277(35):32133–40. doi: 10.1074/jbc.M204507200. [DOI] [PubMed] [Google Scholar]
- 30.Hollander D, Ruble PE., Jr beta-carotene intestinal absorption: bile, fatty acid, pH, and flow rate effects on transport. Am J Physiol. 1978;235(6):E686–91. doi: 10.1152/ajpendo.1978.235.6.E686. [DOI] [PubMed] [Google Scholar]
- 31.Jouni ZE, Wells MA. Purification and partial characterization of a lutein-binding protein from the midgut of the silkworm Bombyx mori. J Biol Chem. 1996;271(25):14722–6. doi: 10.1074/jbc.271.25.14722. [DOI] [PubMed] [Google Scholar]
- 32.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279(47):49447–54. doi: 10.1074/jbc.M405334200. [DOI] [PubMed] [Google Scholar]
- 33.Lakshman MR, Rao MN. Purification and characterization of cellular carotenoid-binding protein from mammalian liver. Methods Enzymol. 1999;299:441–56. doi: 10.1016/s0076-6879(99)99042-0. [DOI] [PubMed] [Google Scholar]
- 34.During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43(7):1086–95. doi: 10.1194/jlr.m200068-jlr200. [DOI] [PubMed] [Google Scholar]
- 35.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135(10):2305–12. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- 36.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271(5248):518–20. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 37.Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J Clin Invest. 1996;98(4):984–95. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sies H, Stahl W. Lycopene: antioxidant and biological effects and its bioavailability in the human. Proc Soc Exp Biol Med. 1998;218(2):121–4. doi: 10.3181/00379727-218-44285a. [DOI] [PubMed] [Google Scholar]
- 39.Ershov Iu V, Dmitrovskii AA, Bykhovskii V. The character of the interaction of beta-carotene-15,15′-dioxygenase from rabbit small intestine with lycopene, 15,15′-dehydro-beta-carotene, lutein, and astaxanthine. Biokhimiia. 1993;58(5):733–9. [PubMed] [Google Scholar]
- 40.Ershov Yu V, Bykhovsky V, Dmitrovskii AA. Stabilization and competitive inhibition of beta-carotene 15,15′-dioxygenase by carotenoids. Biochem Mol Biol Int. 1994;34(4):755–63. [PubMed] [Google Scholar]
- 41.Wyss A. Carotene oxygenases: a new family of double bond cleavage enzymes. J Nutr. 2004;134(1):246S–50S. doi: 10.1093/jn/134.1.246S. [DOI] [PubMed] [Google Scholar]
- 42.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin. A J Biol Chem. 2001;276(17):14110–6. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 43.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′, 10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281(28):19327–38. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136(6):1552–7. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 45.Zaripheh S, Nara TY, Nakamura MT, Erdman JW., Jr Dietary lycopene downregulates carotenoid 15,15′-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr. 2006;136(4):932–8. doi: 10.1093/jn/136.4.932. [DOI] [PubMed] [Google Scholar]
- 46.Zaripheh S, Erdman JW., Jr The biodistribution of a single oral dose of [14C]-lycopene in rats prefed either a control or lycopene-enriched diet. J Nutr. 2005;135(9):2212–8. doi: 10.1093/jn/135.9.2212. [DOI] [PubMed] [Google Scholar]