Abstract
Most hematopoietic malignancies are comprised of cells that are functionally heterogeneous with only a subset being responsible for tumor maintenance. These cancer stem cells are so named because they possess qualities reminiscent of normal tissue stem cells including self-renewal, prolonged survival, and the ability to give rise to cells with more differentiated characteristics. Effort is now focused on identifying cancer stem cells in various hematopoietic malignancies, and defining the cells of origin such that the stepwise accumulation of genetic/epigenetic events necessary for cancer stem cell development can be delineated. A detailed understanding of these processes could lead to development of therapeutics that more effectively treat hematopoietic malignancies and potentially other cancers.
Introduction
Many adult tissues contain stem cells that are able to divide and retain all of their proliferative and developmental potential (self-renewal), while at the same time giving rise to all specialized cells necessary for regeneration (differentiation). The most well characterized tissue-specific stem cells are hematopoietic stem cells. Detailed characterization of hematopoietic stem cells, committed hematopoietic progenitor cells, and differentiated blood cells sets the stage for understanding hematopoietic malignancies1, their cells of origin, and the characteristics of cancer stem cells. Indeed the modern concept of human cancer stem cells initiates from studies in acute myelogenous leukemias2.
The cancer stem cell concept is based on the idea that tumors of a specific tissue often appear to “attempt” to recapitulate the cellular heterogeneity found in the tissues of origin, and thus there are cells in the tumor that are stem-cell like giving rise to the varied cell types. A fundamental test for this hypothesis is whether tumor cells can be separated into those that have the ability to regenerate the tumor, and those that do not possess this ability. This cellular hierarchy has been most clearly demonstrated in acute myelogenous leukemias where some AMLs possess cells with a unique immunophenotype that are able to initiate leukemias in immunodeficient mice whereas most cells are unable to initiate leukemia development2,3. Furthermore the cells that initiate leukemias also give rise to cells that have lost tumor-initiating activity and thus recapitulate the cellular heterogeneity found in the original tumor. Demonstrating this type of functional hierarchy and cellular heterogeneity identifies the presence of a leukemia (or cancer) stem cell as they are presently defined.
Cancer Stem Cells in Myeloid Malignancies
The most detailed characterization of myeloid cell development and stem cell biology has been performed in murine systems, and has informed similar approaches using human cells. Normal hematopoietic stem cells contain self-renewal properties, that are lost as the cells commit to myeloid development, giving rise to committed progenitor cells that while remaining multipotent, no longer possess long-term self-renewal properties (Figure 1). Current studies suggest that human HSC reside in a population of cells that are CD34+/CD38−, and that CD34+, CD38+ cells contain populations that are committed to specific myeloid fates. Initial studies that demonstrated the presence of acute myelogenous leukemia (AML) stem cells delineated the cell surface immunophenotype as CD34+/CD38− thus suggesting similarities between normal and AML stem cells2,3. Further characterization has demonstrated that in many cases the AML stem cells also express antigens such as CD123 (IL3-R) that are not normally found on human HSC4. Also, experimental evidence suggests that AML stem cells may respond differently to specific small molecules (NFKB inhibitors and parthenolide) providing evidence that there may be differences between normal and human stem cells that can be leveraged for therapeutic benefit5. Detailed studies of well-defined murine models of AML also demonstrate phenotypic differences between normal and human stem cells, supporting the possibility AML stem cells have phenotypic, and hopefully functional differences that can be manipulated to specifically target AML stem cells. Studies in chronic myelogenous leukemias (CML) have also been informative as to the properties of human leukemia stem cells. It is widely held that the cancer stem cell in chronic phase CML is similar if not identical to the normal HSC. However recent data suggest that the leukemia stem cell maintaining blast crisis is more consistent with committed hematopoietic progenitors6. Thus it appears that the characteristics of the leukemia stem cell evolve as the leukemia transitions from chronic phase to blast crisis. Future studies using improved model systems and technologies will no doubt improve our understanding of the cellular heterogeneity of myeloid malignancies and hopefully focus our attention on the cells most important to eradicate in patients.
Figure 1. Normal hematopoietic development and acute AML.
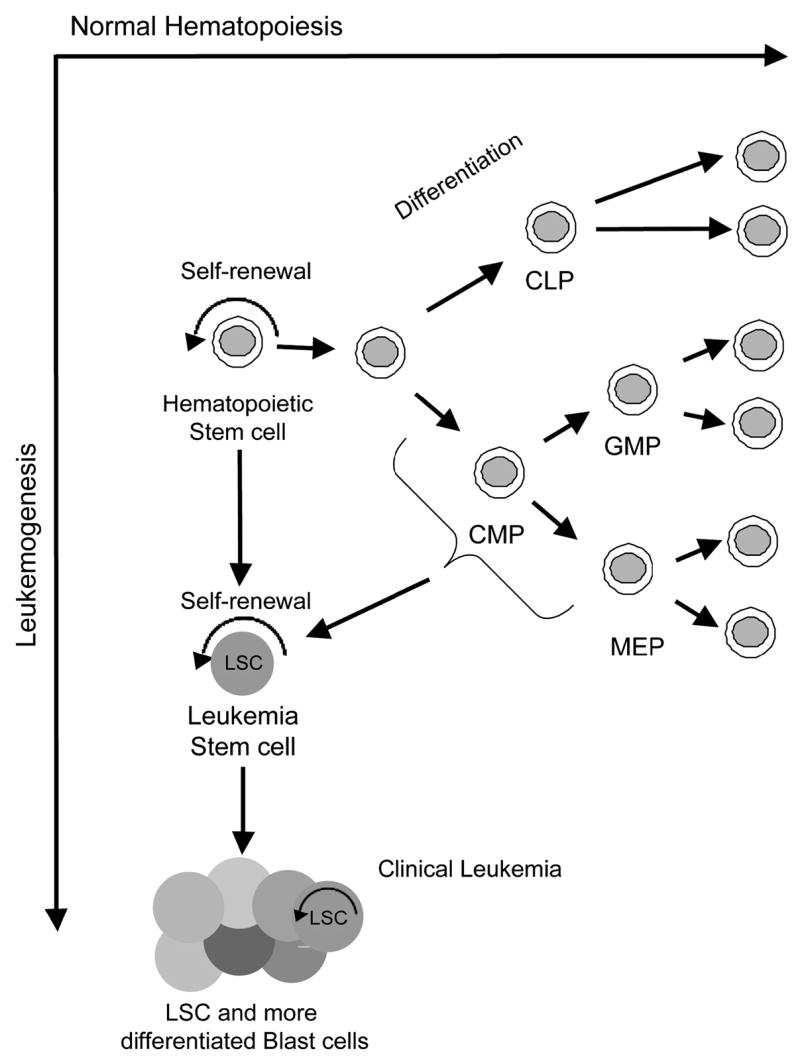
Normal hematopoietic development proceeds from hematopoietic stem through fully differentiated blood cells. A number of cell fate decisions are made during development that give rise to committed hematopoietic progenitors such as common lymphoid progenitors (CLP), common myeloid progenitors (CMP), granulocyte macrophage progenitors (GMP) and megakaryocyte erythroid progenitors (MEP). The only normal cells with self-renewal potential are hematopoietic stem cells. Leukemia stem cells (LSC) arise from hematopoietic stem cells and potentially more committed progenitor cells, and the fully developed AML cells are comprised of a mixture of cells with a hierarchy somewhat similar to normal hematopoiesis. 10
A related but distinct question is what cells are the cell of origin for leukemias and leukemia stem cells. As normal HSC possess indefinite self-renewal properties, and chronic phase CML is likely initiated from HSC, it is clear that normal HSC can give rise to myeloid leukemias. However, the question arises as to whether more committed myeloid cells can give rise to AML (Figure 1). It would appear, based on the studies in blast crises CML, that leukemia stem cells can possess an immunophenotype more consistent with committed myeloid cells, however these leukemias likely progressed from an HSC that harbored the BCR-ABL oncogene. Can AML be directly initiated from a committed myeloid progenitor cell? Recent studies in murine models have demonstrated that leukemias can be initiated from common myeloid progenitors or granulocyte macrophage progenitors7,8. However, only certain fusion oncogenes such as those involving the MLL gene on chromosome 11q23 possess this activity whereas others such as BCR-ABL do not possesses this ability9. The fact that leukemia can be initiated from a cell that lacks intrinsic self-renewal provides an opportunity to determine the pathways responsible for these properties. Ongoing studies will determine if AML can be initiated from human myeloid progenitor cells, and most importantly if leukemias initiated from different cells of origin have different therapeutic outcomes.
Cancer Stem Cells in Lymphoid Malignancies
Self-renewal capacity in most tissues is lost as cells progress through their normal stages of differentiation; for example, myeloid lineage blood cells beyond the level of hematopoietic stem cells no longer possess self-renewal capacity. A notable exception to differentiation-associated loss of self-renewal is the lymphoid system, where self-renewal capacity is preserved until the memory lymphocyte stage in order to maintain life-long immune memory10,11. Somatic hypermutation serves as a marker for the stage of differentiation at which B cell malignancies arise. In general, the presence of somatic hypermutation identifies a tumor as having arisen in germinal center or post-germinal center B cells, while the absence of mutation identifies pre-germinal center B cells. In contrast to myeloid malignancies2,3,12 but consonant with the lineage’s preserved self-renewal capacity, immunoglobulin (Ig) mutation patterns suggest that B cell malignancies can arise from cells throughout the stages of B cell differentiation (Figure 2).
Figure 2. Origin of B cell malignancies in relation to normal B cell differentiation.
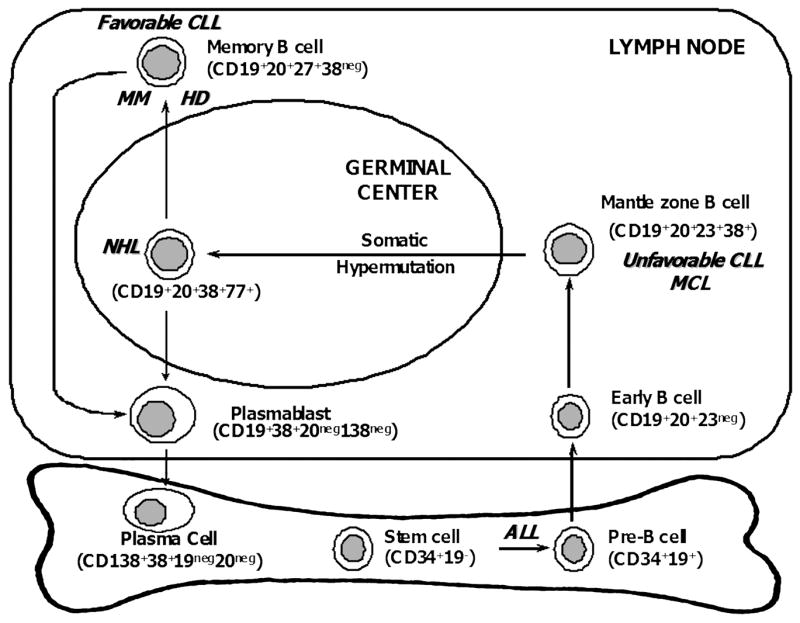
Based on the presence or absence of somatic hypermutation, B cell malignancies (listed in bolded italics) appear to arise at various stages of B cell differentiation: acute lymphocytic leukemia (ALL) from hematopoietic stem cells or pre-B cells, unfavorable chronic lymphocytic leukemia (CLL) and most mantle cell lymphomas (MCL) from the follicular mantle B cells, most other non-Hodgkin’s lymphomas (NHL) from germinal center B cells, and multiple myeloma (MM), Hodgkin’s lymphoma (HL), and favorable CLL from memory B cells.
Multiple myeloma (MM) has generally been considered a disease of malignant plasma cells, and indeed many of clinical consequences of the disease result from the plasma cell bulk. However, normal plasma cells are terminally differentiated and lack self-renewal capacity. Moreover, it has been clear for over 30 years that only a minority of cells from mouse and human MM were clonogenic13,14. Investigators called these rare clonogenic cells “tumor stem cells”13,14. This low clonogenic potential could be explained by either proliferative capacity exclusively restricted to a small subset of cancer cells, or alternatively all the cells within a cancer retaining the capacity to proliferate but only at a low rate. Insufficient tools available at the time precluded investigators from distinguishing which of these two possibilities explained the low clonogenicity of MM. Cells phenotypically resembling mature B cells and sharing Ig gene sequences and idiotype specificity with MM plasma cells have also been found in the marrow and blood of patients with MM15–17. but their role in the pathogenesis of the disease has been unclear. All these data suggest that MM might originate from self-renewing B cells, rather than plasma cells. In fact, it has recently been shown that MM plasma cells actually arise from a small population of self-renewing cancer stem cells that resemble memory B cells.18,19 Not only do these clonotypic B cells circulate in most patients but they also are resistant to many standard anti-MM agents, and thus appear to be responsible for most disease relapses.
Reed-Sternberg (RS) cells, the hallmark of Hodgkin’s lymphoma (HL), are the only blood cells other than plasma cells to occasionally express CD138.20 This has led to the hypothesis that RS cells represent aberrant plasma cell differentiation, supported by data showing that RS cells from HL cell lines expressed a transcriptional profile similar to normal and malignant plasma cells.21 Thus, like plasma cells in MM, RS may not represent HL stem cells. We and others22 found that HL cell lines include a small population of cells that lack the RS markers CD15 and CD30 present on the rest of the cells, while expressing markers consistent with a memory B cell phenotype.23 Moreover, this small subpopulation of phenotypic memory B cells possessed all of the clonogenic capacity within the HL cell lines. We also found that most HL patients, including those with early stage disease, harbor circulating memory B cells with the same clonal Ig gene rearrangement as the patients’ RS cells.23 These data suggest that these clonotypic memory B cells likely represent the HL stem cells. A transforming event in hematopoietic stem cells can produce several different malignancies, chronic myeloid leukemia, myelodysplastic syndrome, acute myeloid leukemia, and probably even acute lymphocytic leuekemia, depending on the degree differentiation associated with the oncogenic hit. Similarly, a transforming event in memory B cells may be able to produce either MM or HL (Figure 3).
Figure 3. Memory B cells as cancer stem cells.
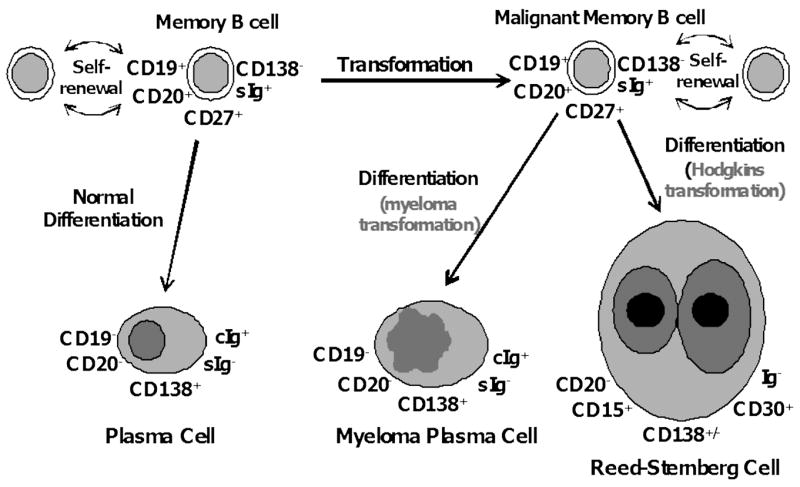
Although not “traditional” stem cells in that they lack multilineage potential, memory B cells can be considered “honorary” stem cells - they are long-lived, self-renew, and differentiate into plasma cells in order to maintain long-term immune memory. A transforming event in memory B cells may be able to produce MM, HL, or CLL, depending on the degree of differentiation associated with the transforming event.
Conclusion
Recent studies have begun to characterize the functional heterogeneity of cells present in various tumors. A discrete subpopulation that has properties similar to normal tissue stem cells can be identified in many hematologic malignancies. Future trials in human hematopoietic malignancies should be focused on whether eradication of the so-called cancer stem cells is critical to a successful therapeutic strategy. If the clinical relevance of cancer stem cells is established, targeting their unique properties should provide opportunities to develop novel therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Jordan CT. Unique molecular and cellular features of acute myelogenous leukemia stem cells. Leukemia. 2002;16:559–62. doi: 10.1038/sj.leu.2402446. [DOI] [PubMed] [Google Scholar]
- 5.Guzman ML, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–9. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 7.Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–22. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 8.Cozzio A, et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–35. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huntly BJ, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–96. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 11.Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RJ, Matsui WH, Smith BD. Cancer stem cells: are we missing the target? J Natl Cancer Inst. 2004;96:583–585. doi: 10.1093/jnci/djh095. [DOI] [PubMed] [Google Scholar]
- 13.Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- 14.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 15.Pilarski LM, Jensen GS. Monoclonal circulating B cells in multiple myeloma. A continuously differentiating, possibly invasive, population as defined by expression of CD45 isoforms and adhesion molecules. Hematol Oncol Clin North Am. 1992;6:297–322. [PubMed] [Google Scholar]
- 16.Billadeau D, Ahmann G, Greipp P, Van Ness B. The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to the malignant plasma cell. J Exp Med. 1993;178:102–1031. doi: 10.1084/jem.178.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szczepek AJ, Seeberger K, Wizniak J, Mant MJ, Belch AR, Pilarski LM. A high frequency of circulating B cells share clonotypic Ig heavy-chain VDJ rearrangements with autologous bone marrow plasma cells in multiple myeloma, as measured by single-cell and in situ reverse transcriptase-polymerase chain reaction. Blood. 1998;92:2844–2855. [PubMed] [Google Scholar]
- 18.Matsui WH, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, Jagannath S, Dhodapkar MV. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbone A, Gloghini A, Gaidano G, Franceschi S, Capello D, Drexler HG, Falini B, Dalla-Favera R. Expression status of BCL-6 and syndecan-1 identifies distinct histogenetic subtypes of Hodgkin’s disease. Blood. 1998;92:2220–2228. [PubMed] [Google Scholar]
- 21.Buettner M, Greiner A, Avramidou A, Jack HM, Niedobitek G. Evidence of abortive plasma cell differentiation in Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma. Hematol Oncol. 2005;23:127–132. doi: 10.1002/hon.764. [DOI] [PubMed] [Google Scholar]
- 22.Newcom SR, Kadin ME, Phillips C. L-428 Reed-Sternberg cells and mononuclear Hodgkin’s cells arise from a single cloned mononuclear cell. Int J Cell Cloning. 1988;6:417–431. doi: 10.1002/stem.5530060606. [DOI] [PubMed] [Google Scholar]
- 23.Jones RJ, Lin L, Gocke C, Hensley K, Siedner M, Barber JP, Kasamon Y, Ambinder RF, Matsui W. Clonotypic B Cells Circulate in Hodgkin’s Lymphoma (HL) 2006;108:470a. [Google Scholar]


