Abstract
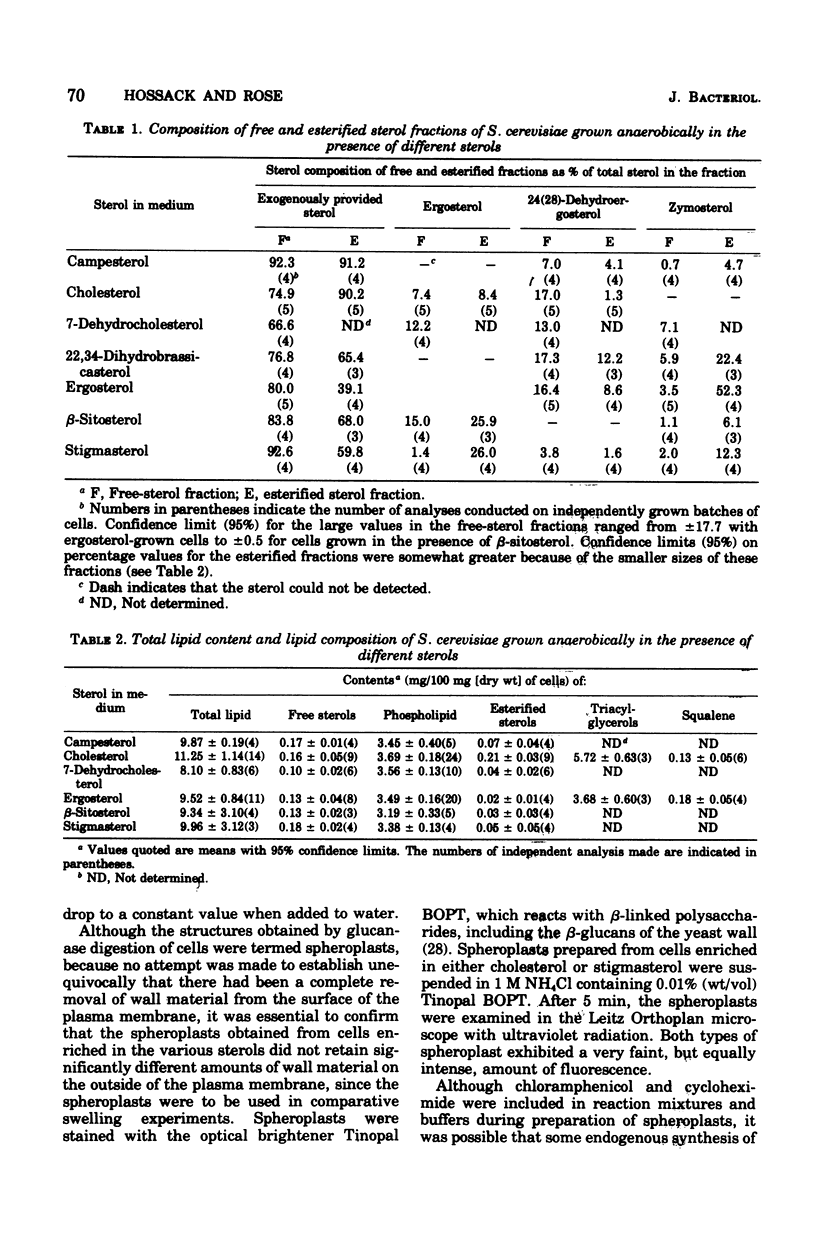
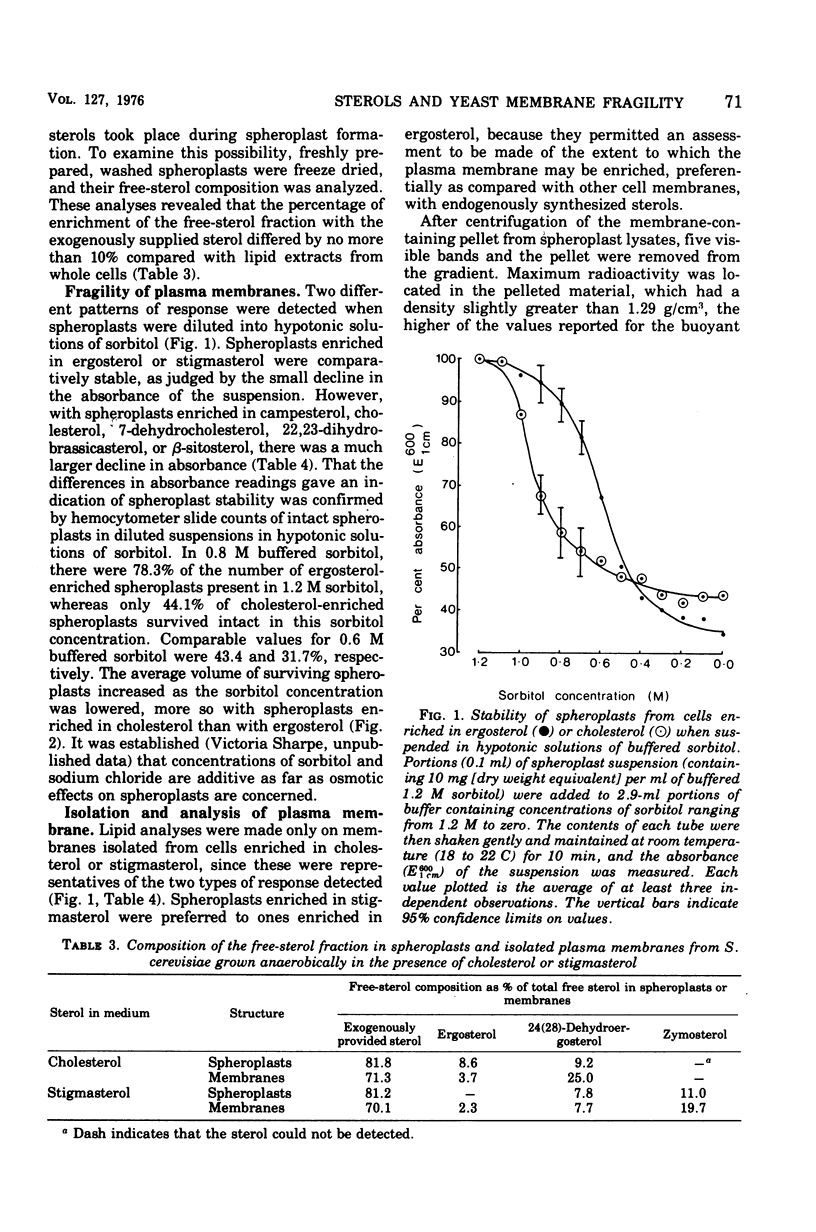
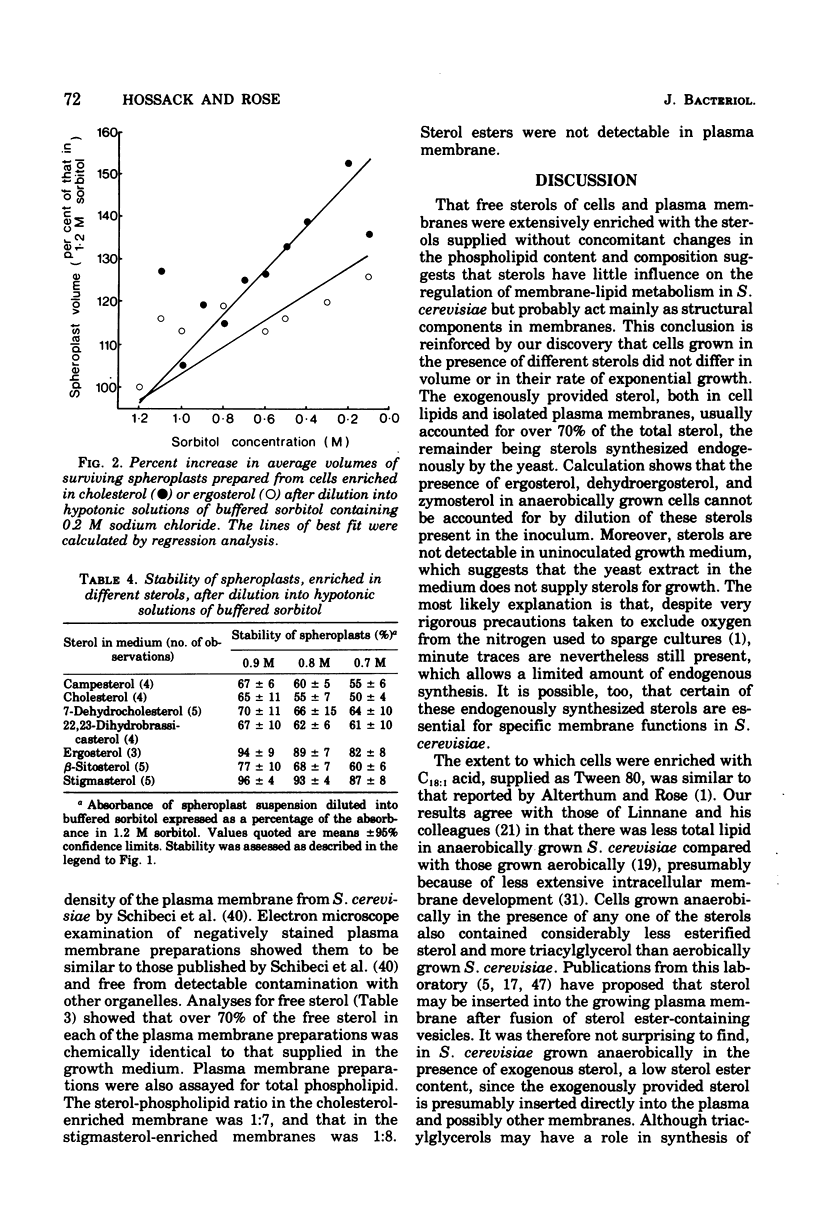
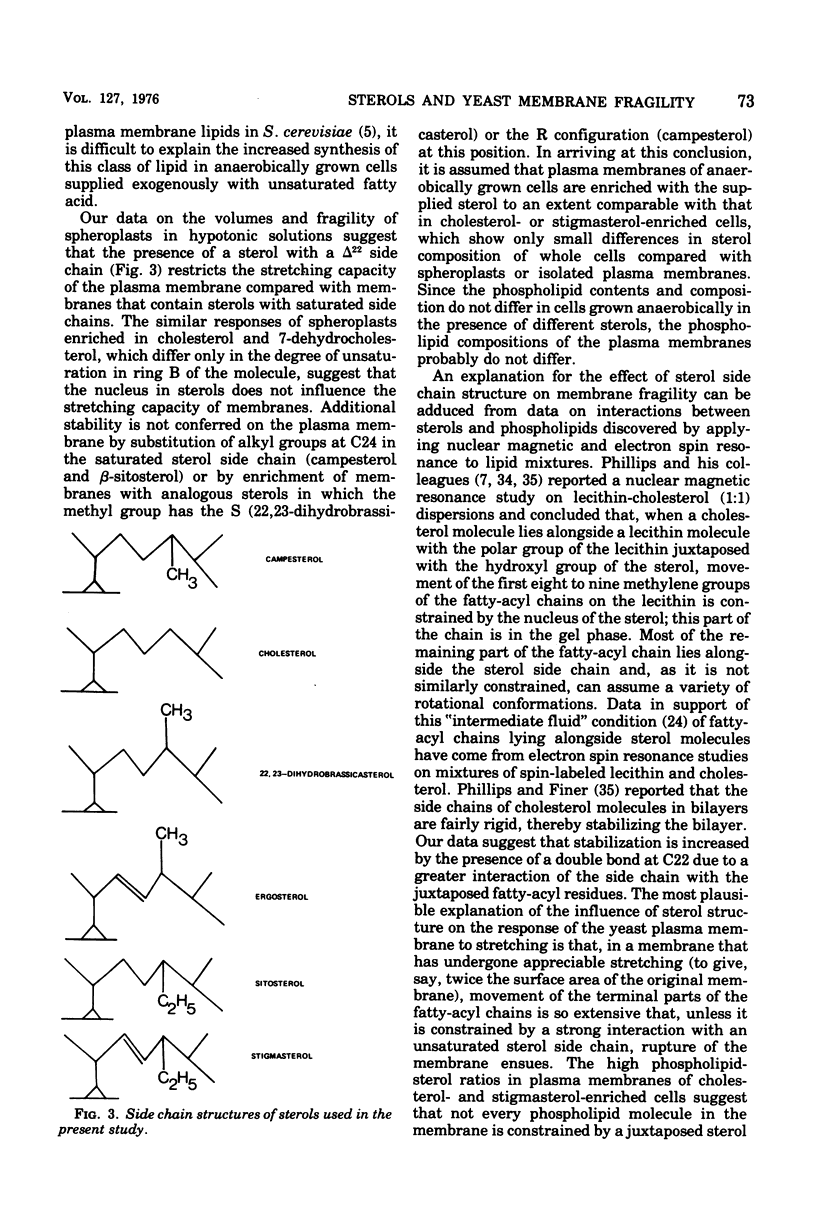
Saccharomyces cerevisiae NCYC 366, grown under strictly anaerobic conditions to induce requirements for an unsaturated fatty acid (supplied by Tween 80) and a sterol, contained free sterol fractions enriched to the extent of 67 to 93% with the exogenously supplied sterol (campesterol, cholesterol, 7-dehydrocholesterol, 22, 23-dihydrobrassicasterol, beta-sitosterol, or stigmasterol). Cells enriched in any one of the sterols did not differ in volume, growth rate, contents of free sterol, esters and phospholipids, or phospholipid composition. Cholesterol-enriched cells contained about 2% more lipid than cells enriched in any of the other sterols, which was largely accounted for by increased contents of triacylglycerols and, to a lesser extent, esterified sterols. Phospholipids were enriched to the extent of about 52 to 63% with C18:1 residues. Cells enriched in ergosterol or stigmasterol were slightly less susceptible to the action of a wall-digesting basidiomycete glucanase than cells enriched with any one of the other sterols. The capacity of the plasma membrane to resist stretching, as indicated by the stability and volume of spheroplasts suspended in hypotonic solutions of buffered sorbitol (particularly in the range 0.9 to 0.7 M), was greater with spheroplasts enriched in sterols with an unsaturated side chain at C17 (ergosterol or stigmasterol) than with any of the other sterols. Plasma membranes were obtained from spheroplasts enriched in cholesterol or stigmasterol and had free sterol fractions containing 70 and 71%, respectively, of the sterol supplied exogenously to the cells. The sterol-phospholipid molar ratios in these membranes were, respectively, 1:7 and 1:8.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Alterthum F., Rose A. H. Osmotic lysis of sphaeroplasts from Saccharomyces cerevisiae grown anaerobically in media containing different unsaturated fatty acids. J Gen Microbiol. 1973 Aug;77(2):371–382. doi: 10.1099/00221287-77-2-371. [DOI] [PubMed] [Google Scholar]
- Bean G. A. Phytosterols. Adv Lipid Res. 1973;11:193–218. [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Nuclear magnetic resonance study of lecithin-cholesterol interactions. J Mol Biol. 1972 Jan 28;63(2):265–279. doi: 10.1016/0022-2836(72)90374-9. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Bruckdorfer K. R., van Deenen L. L. Structural requirements of sterols for the interaction with lecithin at the air water interface. Biochim Biophys Acta. 1972 Jan 17;255(1):311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Diamond R. J., Rose A. H. Osmotic properties of spheroplasts from Saccharomyces cerevisiae grown at different temperatures. J Bacteriol. 1970 May;102(2):311–319. doi: 10.1128/jb.102.2.311-319.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Tinoco J. Monolayer interactions of individual lecithins with natural sterols. Biochim Biophys Acta. 1972 Apr 14;266(1):41–49. doi: 10.1016/0005-2736(72)90117-4. [DOI] [PubMed] [Google Scholar]
- Hunter K., Rose A. H. Lipid composition of Saccharomyces cerevisiae as influenced by growth temperature. Biochim Biophys Acta. 1972 Apr 18;260(4):639–653. doi: 10.1016/0005-2760(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Huotari F. I., Nelson T. E., Smith F., Kirkwood S. Purification of an exo-beta-D-(1 bonded to 3)-glucanase from Basidiomycete species QM 806. J Biol Chem. 1968 Mar 10;243(5):952–956. [PubMed] [Google Scholar]
- Jollow D., Kellerman G. M., Linnane A. W. The biogenesis of mitochondria. 3. The lipid composition of aerobically and anaerobically grown Saccharomyces cerevisiae as related to the membrane systems of the cells. J Cell Biol. 1968 May;37(2):221–230. doi: 10.1083/jcb.37.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHT R. J., LENNARZ W. J., BLOCH K. The metabolism of hydroxystearic acids in yeast. J Biol Chem. 1962 Jun;237:1793–1800. [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- MOORE P. R., BAUMANN C. A. Skin sterols. I. Colorimetric determination of cholesterol and other sterols in skin. J Biol Chem. 1952 Apr;195(2):615–621. [PubMed] [Google Scholar]
- MORPURGO G., SERLUPI-CRESCENZI G., TECCE G., VALENTE F., VENETTACCI D. INFLUENCE OF ERGOSTEROL ON THE PHYSIOLOGY AND THE ULTRA-STRUCTURE OF SACCHAROMYCES CEREVISIAE. Nature. 1964 Feb 29;201:897–899. doi: 10.1038/201897a0. [DOI] [PubMed] [Google Scholar]
- Maeda H., Ishida N. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem. 1967 Aug;62(2):276–278. doi: 10.1093/oxfordjournals.jbchem.a128660. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Finer E. G. The stoichiometry and dynamics of lecithin-cholesterol clusters in bilayer membranes. Biochim Biophys Acta. 1974 Jul 31;356(2):199–206. doi: 10.1016/0005-2736(74)90283-1. [DOI] [PubMed] [Google Scholar]
- Proudlock J. W., Wheeldon L. W., Jollow D. J., Linnane A. W. Role of sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Mar 4;152(2):434–437. doi: 10.1016/0005-2760(68)90060-x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Engelman D. M. Molecular mechanism for the interaction of phospholipid with cholesterol. Nat New Biol. 1972 May 10;237(71):42–44. doi: 10.1038/newbio237042a0. [DOI] [PubMed] [Google Scholar]
- Schibeci A., Rattray J. B., Kidby D. K. Isolation and identification of yeast plasma membrane. Biochim Biophys Acta. 1973 Jun 7;311(1):15–25. doi: 10.1016/0005-2736(73)90250-2. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., van Dijck P. W., Godlbach R. W., Demel R. A., van Deenen L. L. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Dec 22;330(3):269–282. doi: 10.1016/0005-2736(73)90232-0. [DOI] [PubMed] [Google Scholar]
- van DEENEN L., HOUTSMULLERUM, de HASS G., MULDER E. Monomolecular layers of synthetic phosphatides. J Pharm Pharmacol. 1962 Jul;14:429–444. doi: 10.1111/j.2042-7158.1962.tb11121.x. [DOI] [PubMed] [Google Scholar]
- van Deenen L. L. Some structural and dynamic aspects of lipids in biological membranes. Ann N Y Acad Sci. 1966 Jul 14;137(2):717–730. doi: 10.1111/j.1749-6632.1966.tb50193.x. [DOI] [PubMed] [Google Scholar]



