Abstract
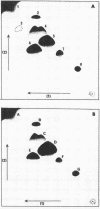
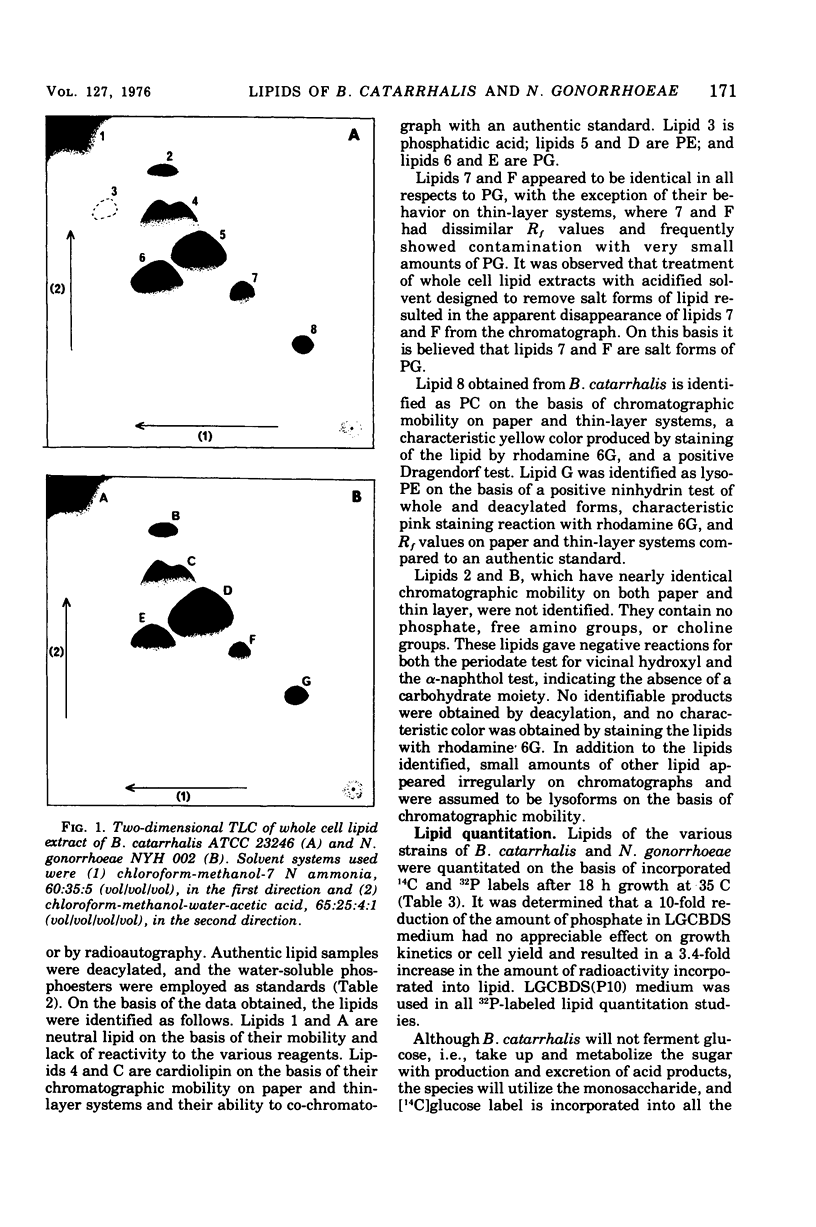
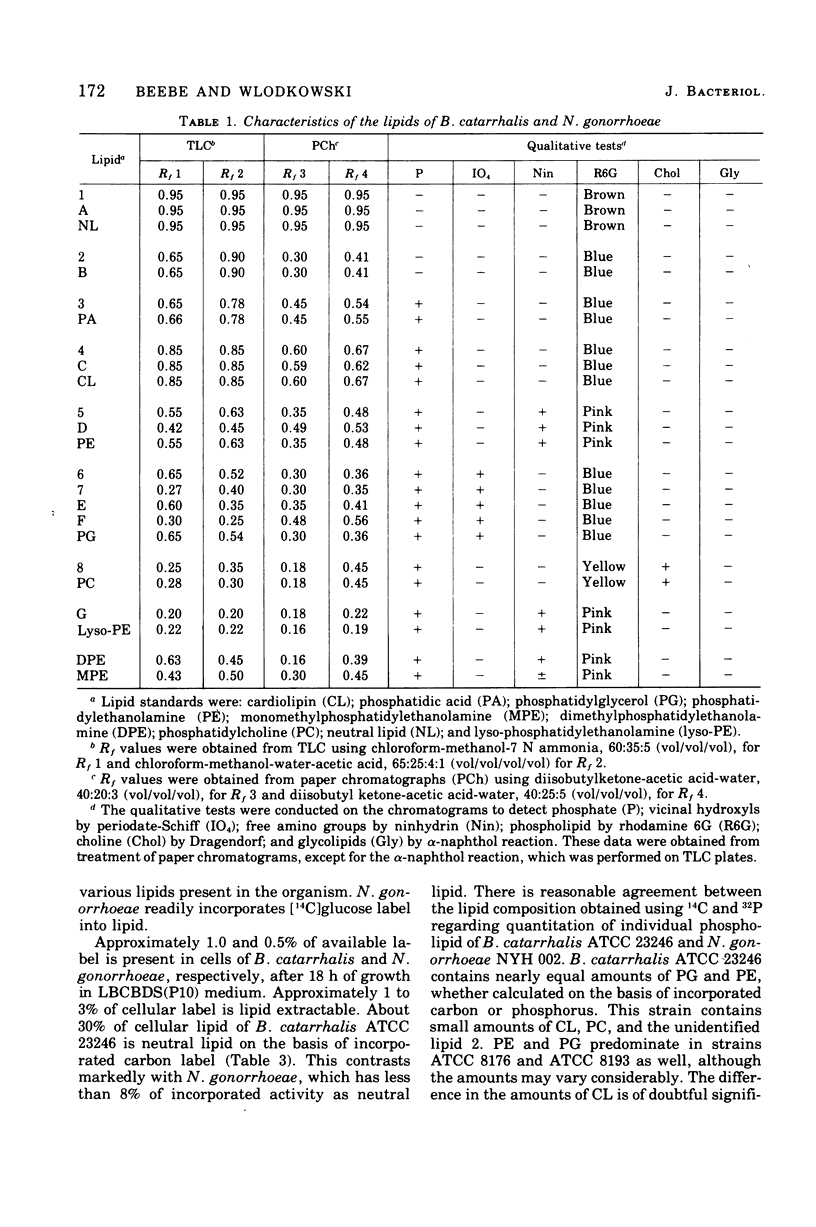
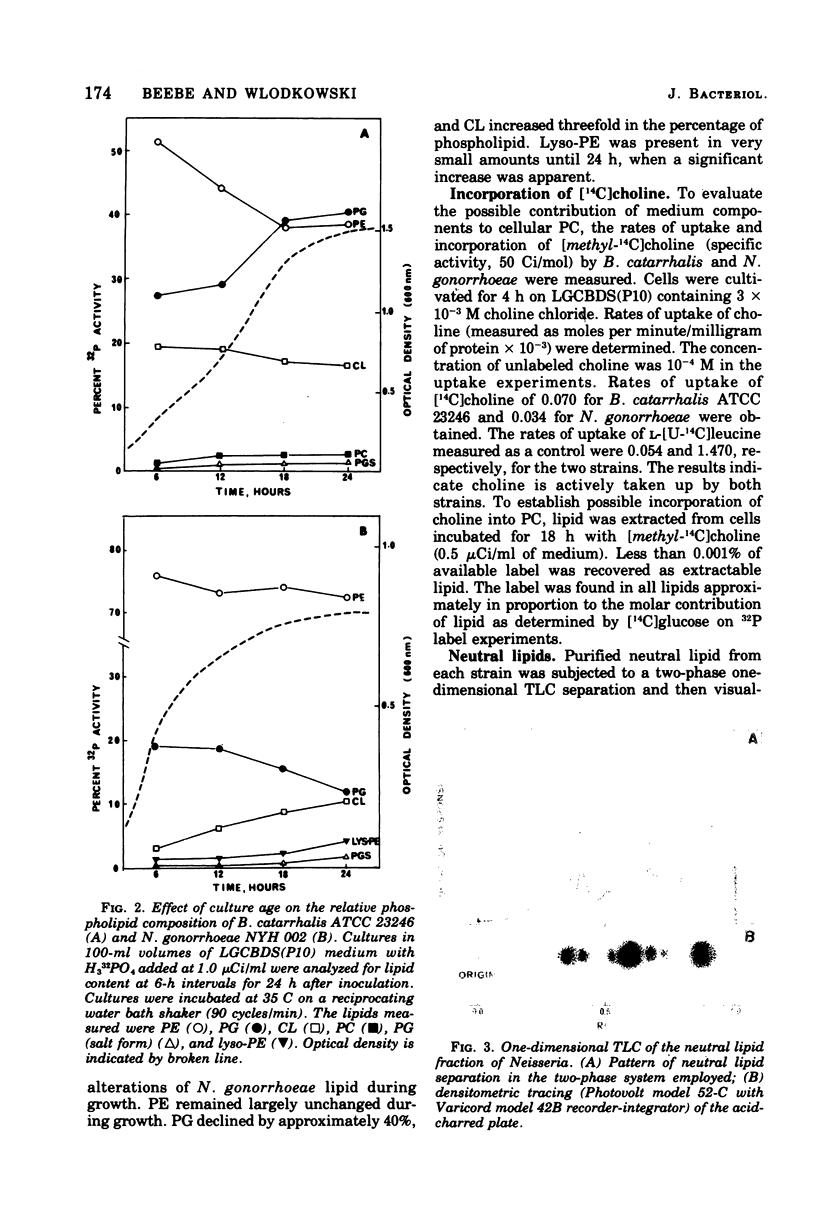
Three strains of Branhamella catarrhalis and three strains of Neisseria gonorrhoeae were analyzed with regard to their phospholipid and neutral lipid composition. B. catarrhalis (ATCC 23246) contained 5.12 +/- 0.34% lipid, determined gravimetrically, compared to 8.56 +/- 0.15% and 9.73 +/- 0.06% for two strains of N. gonorrhoeae. Cardiolipin, phosphatidylglycerol, and phosphatidyl-ethanolamine were identified in extracts of both species. In addition, B. catarrhalis contained small amounts of phosphatidylcholine, and N. gonorrhoeae contained small amounts of lyso-phosphatidylethanolamine, which accumulated with autolysis accompanying late cell culture growth. The kinetics of change of relative amounts of phospholipids in both species were measured and found to differ substantially. Neutral lipid accounted for 30.4% of the total lipid of B. catarrhalis (ATCC 23246) and 7.6% of the total lipid of N. gonorrhoeae NYH 002. Hydrocarbons, triglycerides, free fatty acids, coenzyme Q, diglycerides, and free hydroxy fatty acids were identified in the neutral lipid fraction of both species. The three strains of N. gonorrhoeae, sensitive, intermediate, and resistant to penicillin, exhibited no significant difference in the composition or metabolism of phospholipid.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON A. A., MARUO B. Piant phospholipids. I. Identification of the phosphatidyl glycerols. Biochim Biophys Acta. 1958 Jan;27(1):189–195. doi: 10.1016/0006-3002(58)90308-1. [DOI] [PubMed] [Google Scholar]
- Beebe J. L., Umbreit W. W. Extracellular lipid of Thiobacillus thiooxidans. J Bacteriol. 1971 Oct;108(1):612–614. doi: 10.1128/jb.108.1.612-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. B., Kellogg D. S., Thacker L., Turner E. M. Analysis by gas chromatography of hydroxy acids produced by several species of Neisseria. Can J Microbiol. 1972 Feb;18(2):157–168. doi: 10.1139/m72-026. [DOI] [PubMed] [Google Scholar]
- Card G. L., Georgi C. E., Militzer W. E. Phospholipids from Bacillus stearothermophilus. J Bacteriol. 1969 Jan;97(1):186–192. doi: 10.1128/jb.97.1.186-192.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., DITTMER J. C., WARASHINA E. A column chromatographic separation of classes of phospholipides. J Biol Chem. 1957 Oct;228(2):685–700. [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975 May;122(2):385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten E. 6-Phosphogluconate dehydrogenase and enzymes of the Entner-Doudoroff pathway in Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):207–213. doi: 10.1111/j.1699-0463.1974.tb02313.x. [DOI] [PubMed] [Google Scholar]
- Holten E. Glucokinase and glucose 6-phosphate dehydrogenase in Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):201–206. doi: 10.1111/j.1699-0463.1974.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Holten E. Glutamate dehydrogenases in genus Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):49–58. doi: 10.1111/j.1699-0463.1973.tb02186.x. [DOI] [PubMed] [Google Scholar]
- Ikawa M. Bacterial phosphatides and natural relationships. Bacteriol Rev. 1967 Mar;31(1):54–64. doi: 10.1128/br.31.1.54-64.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACIN H., MISHKIN A. R. SEPARATION OF CARBOHYDRATES ON BORATE-IMPREGNATED SILICA GEL G PLATES. J Chromatogr. 1965 Apr;18:170–173. doi: 10.1016/s0021-9673(01)80341-1. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., HATEFI Y., WIDMER C., CRANE F. L. Studies on the electron transport system. XX. Chemical and physical properties of the coenzyme Q family of compounds. Biochim Biophys Acta. 1959 May;33(1):169–185. doi: 10.1016/0006-3002(59)90511-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert M. A., Hollis D. G., Moss C. W., Weaver R. E., Thomas M. L. Cellular fatty acids of nonpathogenic Neisseria. Can J Microbiol. 1971 Dec;17(12):1491–1502. doi: 10.1139/m71-239. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Kellogg D. S., Jr, Farshy D. C., Lambert M. A., Thayer J. D. Cellular fatty acids of pathogenic Neisseria. J Bacteriol. 1970 Oct;104(1):63–68. doi: 10.1128/jb.104.1.63-68.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W., Saz A. K. Possible mechanism of decreased susceptibility of Neisseria gonorrhoeae to penicillin. Antimicrob Agents Chemother. 1975 Jun;7(6):788–792. doi: 10.1128/aac.7.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes W., Keith A., Wanda P. Active transport of choline by a marine pseudomonad. J Bacteriol. 1974 Oct;120(1):197–202. doi: 10.1128/jb.120.1.197-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokinger H. E., Ackerman H., Carpenter C. M. Studies on the gonococcus: I. Constituents of the cell. J Bacteriol. 1944 Feb;47(2):129–139. doi: 10.1128/jb.47.2.129-139.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Phospholipids and fatty acids of Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):713–717. doi: 10.1128/jb.124.2.713-717.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J. The preservation of gonococci in liquid nitrogen. J Clin Pathol. 1971 Mar;24(2):122–123. doi: 10.1136/jcp.24.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAKAWA T., UETA N. GASCHROMATOGRAPHIC STUDIES OF MICROBIAL COMPONENTS. I. CARBOHYDRATE AND FATTY ACID CONSTITUTION OF NEISSERIA. Jpn J Exp Med. 1964 Dec;34:361–374. [PubMed] [Google Scholar]