Abstract
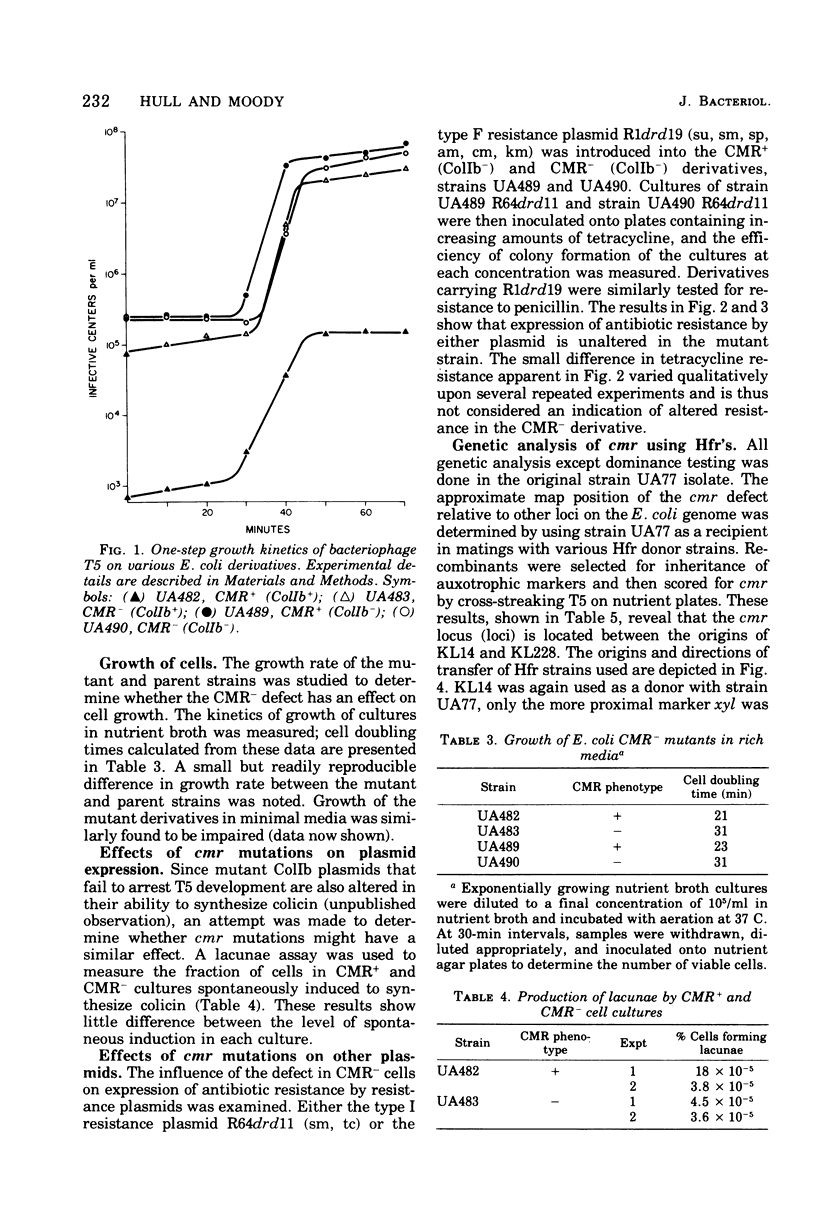
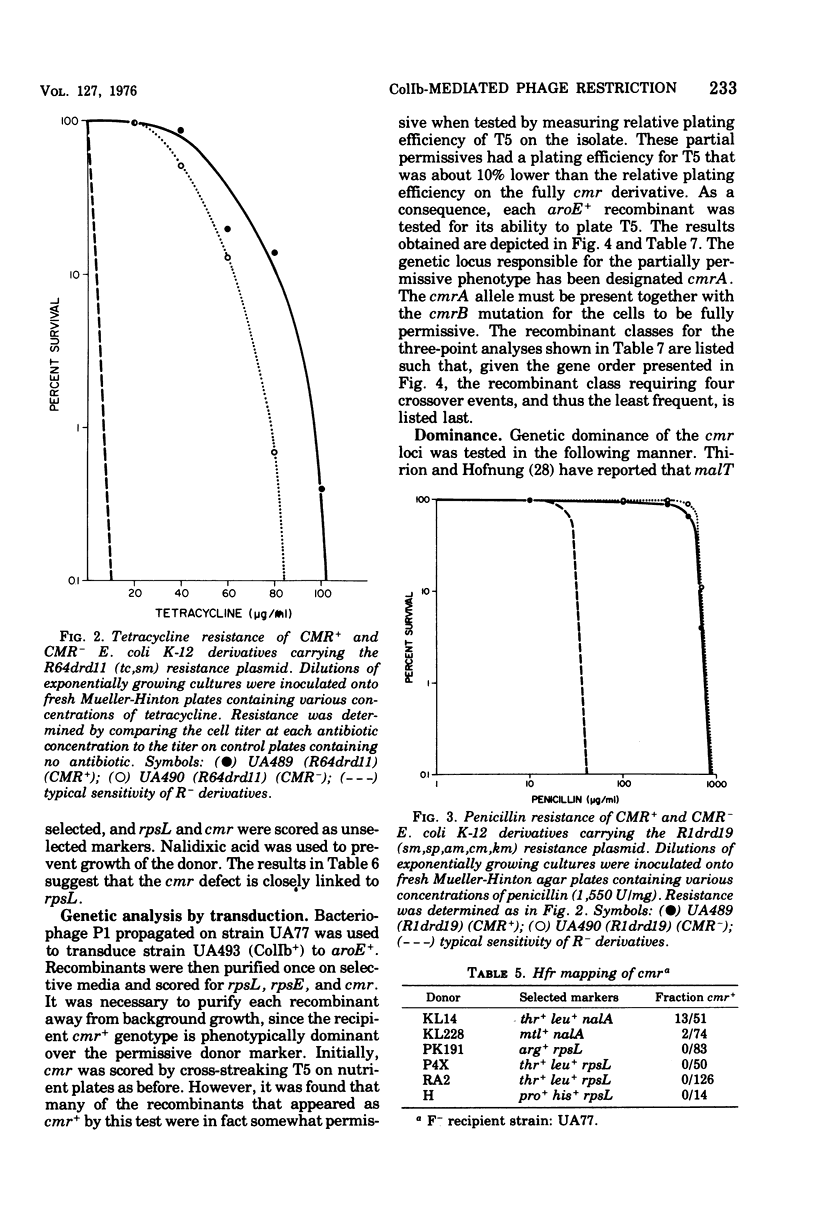
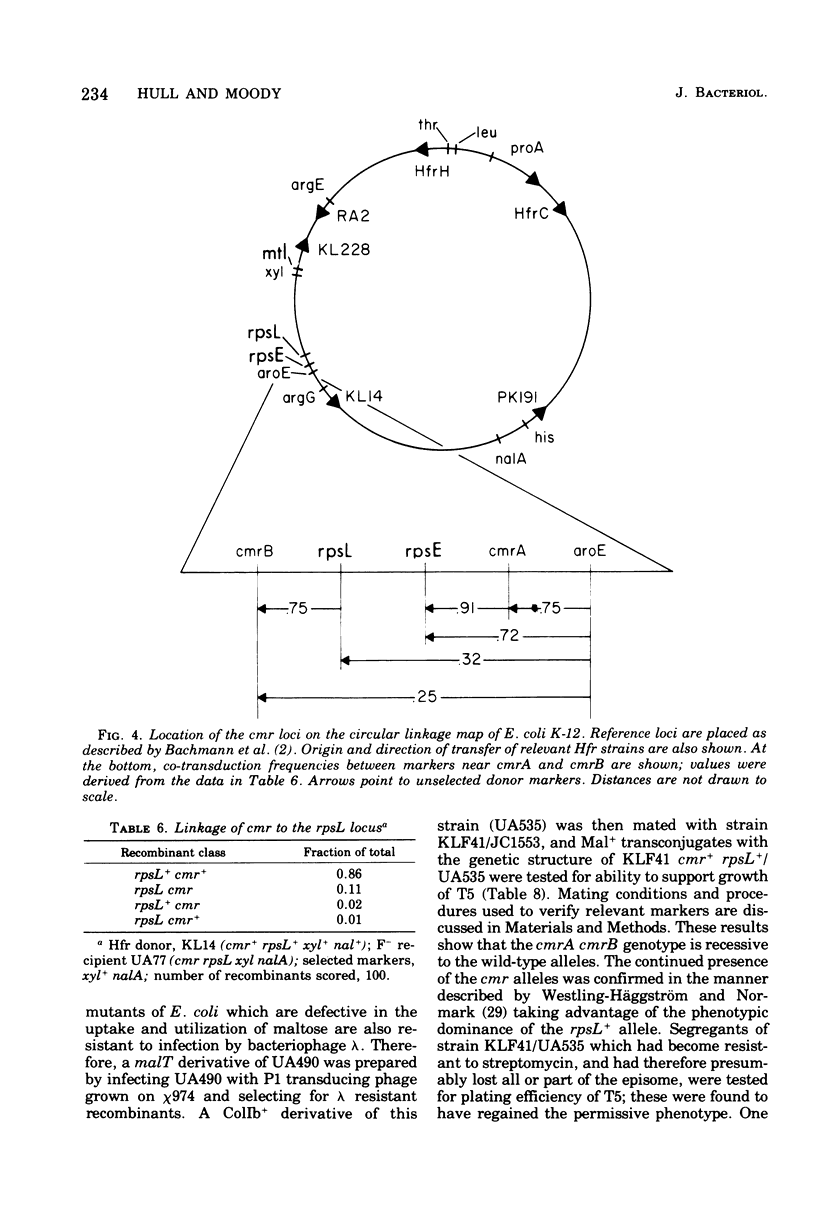
A mutant derivative of Escherichia coli K-12 has been isolated which is permissive for bacteriophage T5 infection even when harboring a wild-type ColIb plasmid. The fully permissive phenotype was the result of two mutations that are located near the rpsL-rpsE region on the E. coli chromosome and are recessive to the wild-type alleles. These mutations had little or no effect on induction of colicin synthesis and did not affect the expression of antibiotic resistance by the resistance plasmids R64drd11 or R1drd19. Cells harboring the mutant alleles grew more slowly than isogenic wild-type derivatives in either minimal or complete media.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Mabie C. T., Malamy M. H. T7 protein synthesis in F-factor-containing cells: evidence for an episomally induced impairment of translation and relation to an alteration in membrane permeability. J Virol. 1975 Jan;17(1):94–105. doi: 10.1128/jvi.17.1.94-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chace K. V., Hall D. H. Characterization of new regulatory mutants of bacteriophage T4. II. New class of mutants. J Virol. 1975 Apr;15(4):929–945. doi: 10.1128/jvi.15.4.929-945.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. G. Colicinogeny and related phenomena. Bacteriol Rev. 1975 Dec;39(4):464–515. doi: 10.1128/br.39.4.464-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Moyer R. W. In vivo repair of bacteriophage t5 DNA: an assay for viral growth control. Virology. 1975 Aug;66(2):393–407. doi: 10.1016/0042-6822(75)90212-3. [DOI] [PubMed] [Google Scholar]
- Ilyina T. S., Ovadis M. I., Mindlin S. Z., Gorlenko Z. M., Khesin R. B. Interaction of RNA polymerase mutations in haploid and merodiploid cells of Escherichia coli K-12. Mol Gen Genet. 1971;110(2):118–133. doi: 10.1007/BF00332643. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Konisky J. Studies of the regulation of colicin Ib synthesis. II. The synthesis of col Ib-P9 specific RNA in vivo. Mol Gen Genet. 1974;132(3):223–232. doi: 10.1007/BF00269395. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Burgess R. R., Nomura M. Identification of a gene for the alpha-subunit of RNA polymerase at the str-spc region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5036–5040. doi: 10.1073/pnas.72.12.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONK M., CLOWES R. C. THE REGULATION OF COLICIN SYNTHESIS AND COLICIN FACTOR TRANSFER IN ESCHERICHIA COLI K12. J Gen Microbiol. 1964 Sep;36:385–392. doi: 10.1099/00221287-36-3-385. [DOI] [PubMed] [Google Scholar]
- MONK M., CLOWES R. C. TRANSFER OF THE COLICIN I FACTOR IN ESCHERICHIA COLI K12 AND ITS INTERACTION WITH THE F FERTILITY FACTOR. J Gen Microbiol. 1964 Sep;36:365–384. doi: 10.1099/00221287-36-3-365. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- McCorquodale D. J., Lanni Y. T. Patterns of protein synthesis in Escherichia coli infected by amber mutants in the first-step-transfer DNA of T5. J Mol Biol. 1970 Feb 28;48(1):133–143. doi: 10.1016/0022-2836(70)90224-x. [DOI] [PubMed] [Google Scholar]
- Miles A. A. Muriel Robertson, 1883-1973. J Gen Microbiol. 1976 Jul;95(1):1–8. doi: 10.1099/00221287-95-1-1. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K., McCorquodale D. J. Abortive infection by bacteriophage BF23 due to the colicin Ib factor. II. Involvement of pre-early proteins. J Mol Biol. 1974 May 5;85(1):67–74. doi: 10.1016/0022-2836(74)90129-6. [DOI] [PubMed] [Google Scholar]
- Moody E. E., Hayes W. Chromosome transfer by autonomous transmissible plasmids: the role of the bacterial recombination (rec) system. J Bacteriol. 1972 Jul;111(1):80–85. doi: 10.1128/jb.111.1.80-85.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer R. W., Fu A. S., Szabo C. Regulation of bacteriophage T5 development by ColI factors. J Virol. 1972 May;9(5):804–812. doi: 10.1128/jvi.9.5.804-812.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Ozeki H. Early abortive lysis by phage BF23 in Escherichia coli K-12 carrying the colicin Ib factor. J Virol. 1968 Nov;2(11):1249–1254. doi: 10.1128/jvi.2.11.1249-1254.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbasku D. A., Buchanan J. M. Patterns of ribonucleic acid synthesis in T5-infected Escherichia coli. II. Separation of high molecular weight ribonucleic acid species by disc electrophoresis on acrylamide gel columns. J Biol Chem. 1970 May 25;245(10):2679–2692. [PubMed] [Google Scholar]
- Strobel M., Nomura M. Restriction of the growth of bacteriophage BF23 by a colicine I (Col I-P9) factor. Virology. 1966 Apr;28(4):763–765. doi: 10.1016/0042-6822(66)90263-7. [DOI] [PubMed] [Google Scholar]
- Szabo C., Dharmgrongartama B., Moyer R. W. The regulation of transcription in bacteriophage T5-infected Escherichia coli. Biochemistry. 1975 Mar 11;14(5):989–997. doi: 10.1021/bi00676a018. [DOI] [PubMed] [Google Scholar]
- Thirion J. P., Hofnung M. On some genetic aspects of phage lambda resistance in E. coli K12. Genetics. 1972 Jun;71(2):207–216. doi: 10.1093/genetics/71.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling-Häggström B., Normark S. Genetic and physiological analysis of an envB spherelike mutant of Escherichia coli K-12 and characterization of its transductants. J Bacteriol. 1975 Jul;123(1):75–82. doi: 10.1128/jb.123.1.75-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]



