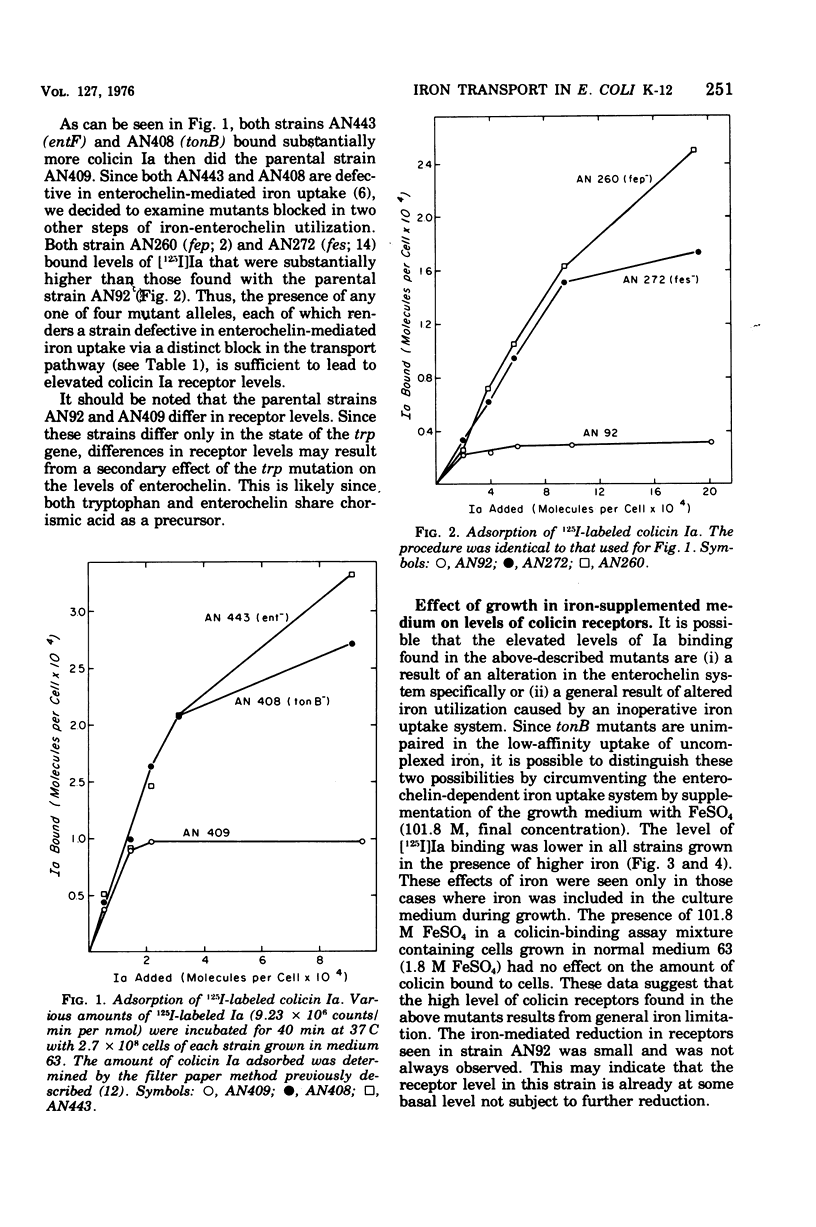
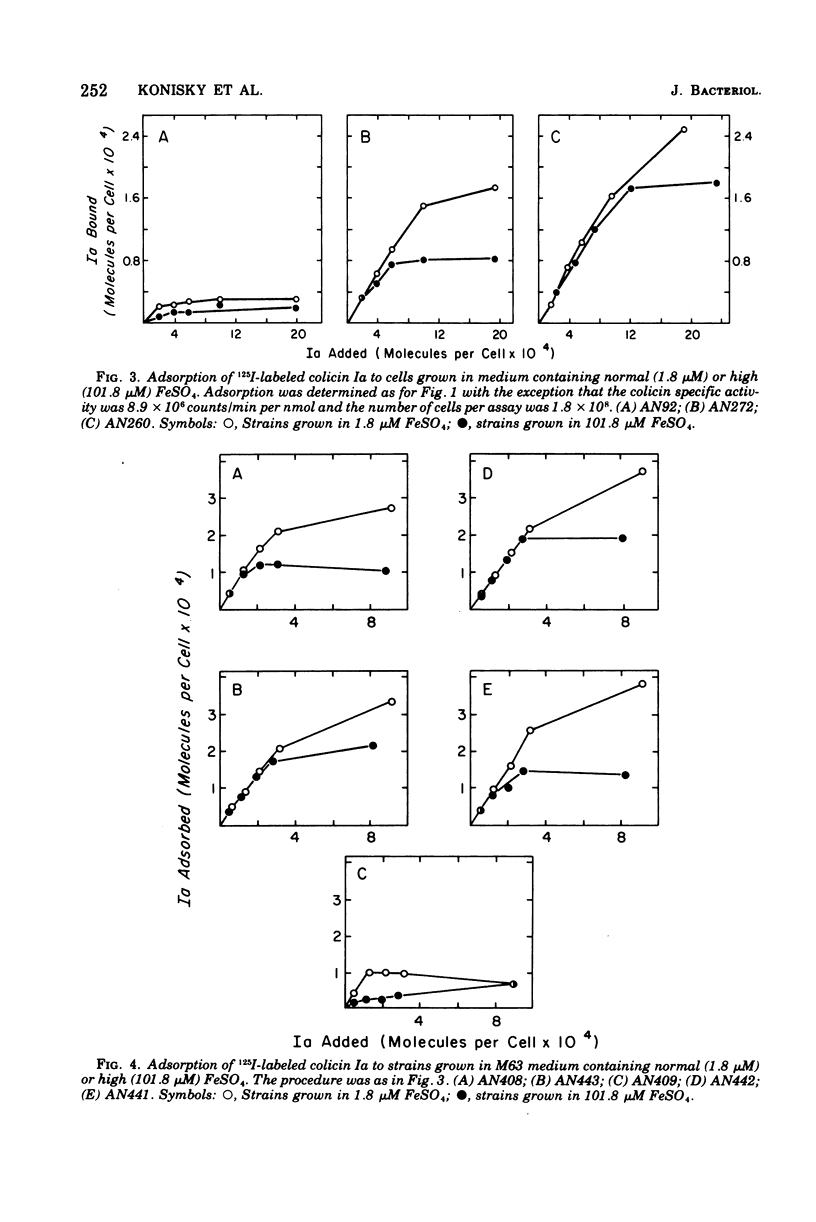
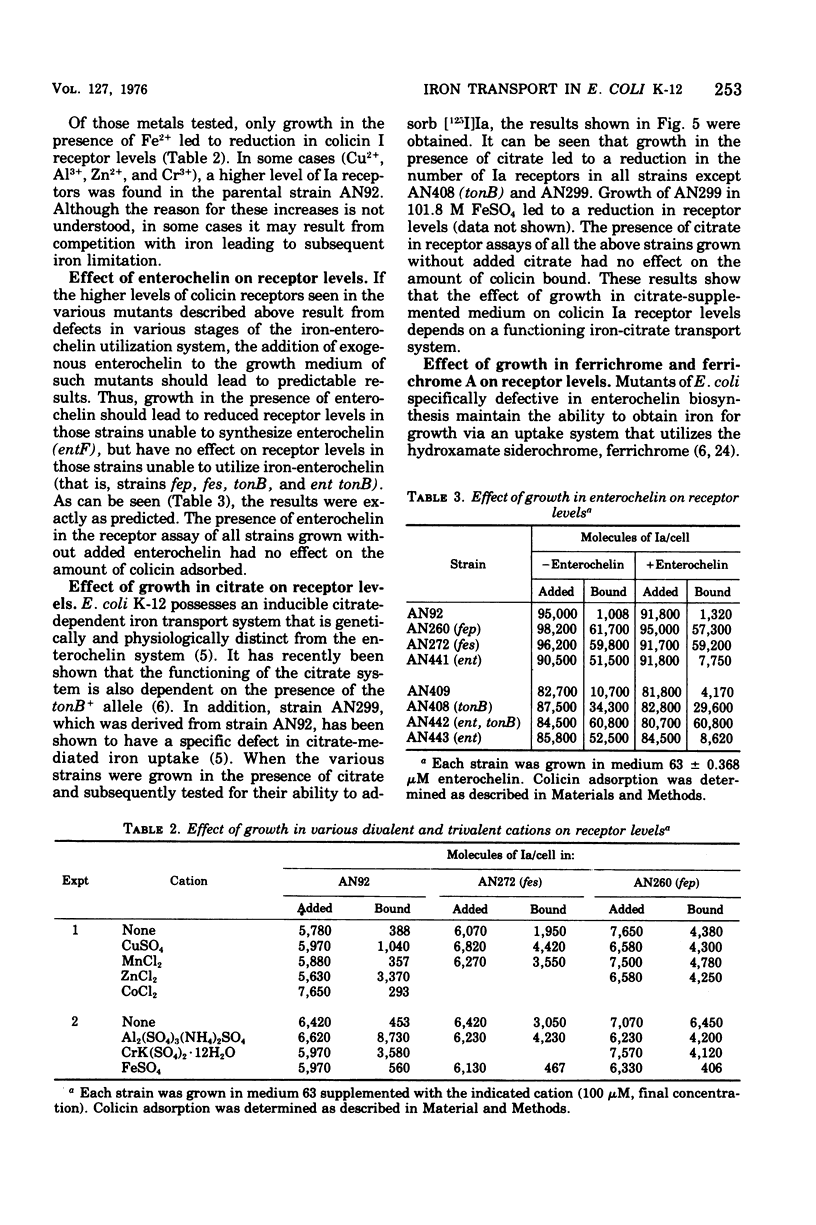
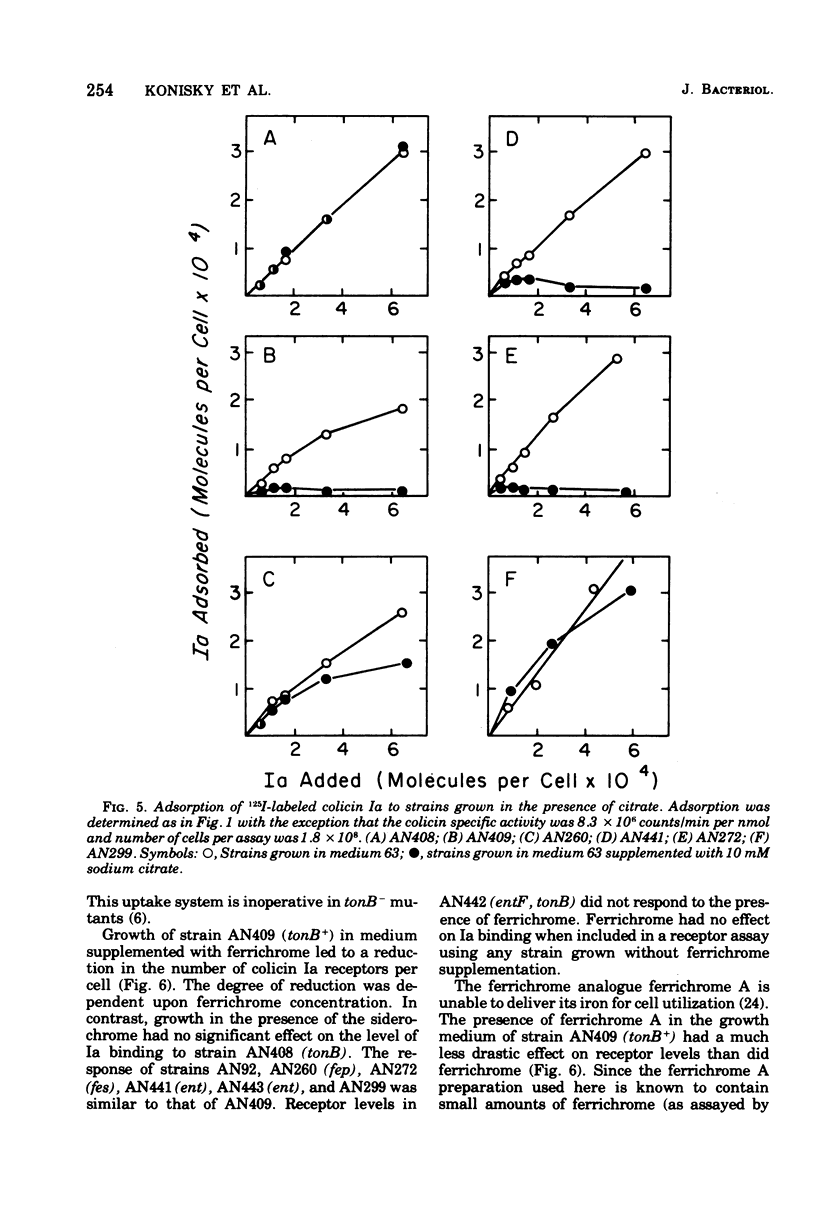
Abstract
Strains of Escherichia coli K-12 defective in their ability to utilize exogenously supplied iron due to genetic defects in the entF, tonB, fes, or fep gene exhibited elevated levels of the specific outer-membrane receptor for colicin Ia when compared with parental strains. Although entF, fes, and fep strains showed a higher degree of Ia sensitivity than did the parental strains, tonB strains were resistant to colicin action. The colicin insensitivity of tonB strains was not due to hyperproduction of enterochelin. Growth in medium containing 101.8 muM Fe2+ led to a lowering of receptor levels in all the above strains and resulted in decreased colicin Ia sensitivity in all strains except tonB, which was already at maximal resistance. Growth in citrate plus iron (1.8 muM) or in ferrichrome resulted in a substantial reduction in both receptor levels and Ia sensitivity in ent, fes, and fep strains but had no effect on receptor levels in tonB strains. Growth in citrate did not lead to an alteration in receptor levels in a mutant specifically defective in citrate-mediated iron transport. The presence of enterochelin during growth led to a reduction in the number of receptors in the parental and ent strains but not in tonB, fes, or fep strains. Thus, in all cases examined, there was an inverse relationship between the number of colicin receptors per cell and the ability of the strain to take up iron from the growth medium. This suggests that under conditions of iron limitation there is a derepression of colicin Ia receptor biosynthesis. These results may point to a role of the colicin I receptor in iron uptake.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bharier M. A., Rittenberg S. C. Chemistry of axial filaments of Treponema zuezerae. J Bacteriol. 1971 Jan;105(1):422–429. doi: 10.1128/jb.105.1.422-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group B. J Bacteriol. 1975 Jul;123(1):96–101. doi: 10.1128/jb.123.1.96-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer D. N., Davis W. B., Byers B. R. Repression of phenolic acid-synthesizing enzymes and its relation to iron uptake in Bacillus subtilis. J Bacteriol. 1970 Jan;101(1):181–187. doi: 10.1128/jb.101.1.181-187.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Gilchrist M. J., Konisky J. Lowered levels of colicin Ia membrane receptors in an Escherichia coli mutant defective in heme biosynthesis. J Bacteriol. 1976 Mar;125(3):1223–1225. doi: 10.1128/jb.125.3.1223-1225.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K. Colicin B: mode of action and inhibition by enterochelin. J Bacteriol. 1973 Jun;114(3):1217–1224. doi: 10.1128/jb.114.3.1217-1224.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. A function common to iron-enterochelin transport and action of colicins B, I, V in Escherichia coli. FEBS Lett. 1975 Nov 15;59(2):277–281. doi: 10.1016/0014-5793(75)80392-9. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Konisky J., Cowell B. S., Gilchrist M. J. Colicin Ia and Ib binding to Escherichia coli envelopes and partially purified cell walls. J Supramol Struct. 1973;1(3):208–219. doi: 10.1002/jss.400010306. [DOI] [PubMed] [Google Scholar]
- Konisky J., Cowell B. S. Interaction of colicin Ia with bacterial cells. Direct measurement of Ia-receptor interaction. J Biol Chem. 1972 Oct 25;247(20):6524–6529. [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Luke R. K., Gibson F. Location of three genes concerned with the conversion of 2,3-dihydroxybenzoate into enterochelin in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):557–562. doi: 10.1128/jb.107.2.557-562.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray J. W., Jr, Herrmann K. M. Derepression of certain aromatic amino acid biosynthetic enzymes of Escherichia coli K-12 by growth in Fe3+-deficient medium. J Bacteriol. 1976 Feb;125(2):608–615. doi: 10.1128/jb.125.2.608-615.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Frick K., Neilands J. B. Siderophore protection against colicins M, B, V, and Ia in Escherichia coli. J Bacteriol. 1976 Apr;126(1):7–12. doi: 10.1128/jb.126.1.7-12.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltzien H. U., Jesaitis M. A. The nature of the cilicin K receptor of Escherichia coli Cullen. J Exp Med. 1971 Mar 1;133(3):534–553. doi: 10.1084/jem.133.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]


