Abstract
Norepinephrine contributes to antinociceptive, sedative, and sympatholytic responses in vivo, and α2 adrenergic receptor (α2AR) agonists are used clinically to mimic these effects. Lack of subtype-specific agonists has prevented elucidation of the role that each α2AR subtype (α2A, α2B, and α2C) plays in these central effects. Here we demonstrate that α2AR agonist-elicited sedative, anesthetic-sparing, and analgesic responses are lost in a mouse line expressing a subtly mutated α2AAR, D79N α2AAR, created by two-step homologous recombination. These functional changes are accompanied by failure of the D79N α2AAR to inhibit voltage-gated Ca2+ currents and spontaneous neuronal firing, a measure of K+ current activation. These results provide definitive evidence that the α2AAR subtype is the primary mediator of clinically important central actions of α2AR agonists and suggest that the D79N α2AAR mouse may serve as a model for exploring other possible α2AAR functions in vivo.
α2-adrenergic receptors (α2ARs) present in the central nervous system (CNS) respond to norepinephrine (NE) and epinephrine and mediate sympatholytic, sedative-hypnotic, analgesic, anesthetic-sparing, hypotensive, and anxiolytic responses (1). Many of these responses are therapeutically useful and are exploited clinically, for example, during anesthesia and to attenuate the symptoms of opioid withdrawal (2). Three α2AR subtypes have been revealed by pharmacological (α2AAR, α2BAR, and α2CAR) and molecular cloning (α2aAR, α2bAR, and α2cAR) strategies (3), and all couple, via pertussis toxin-sensitive Gi/Go proteins, to attenuation of adenylyl cyclase, suppression of voltage-gated Ca2+ channels, and activation of inwardly rectifying K+ channels (4).
Multiple experimental limitations have precluded clarifying the involvement of each α2AR subtype in catecholamine-mediated physiological responses in the CNS. Subtype-specific α2AR agonists and antagonists are not available (5); even when subtype selectivity has been noted in vitro, varying and unknown in vivo bioavailability precludes confident correlation of the administered dose with the amount of drug at the receptor site. Previous studies to explore α2AR involvement in various responses have used prazosin to block catecholamine responses mediated by α1 adrenergic receptors (α1AR); however, it is now known that the α2BAR and α2CAR subtypes also are blocked by prazosin (5), thus confounding the interpretations of these earlier studies. In addition, because α1AR can functionally antagonize α2AR-mediated responses in some settings, α2AR responses in the presence of prazosin (added to block α1AR, α2BAR, and α2CAR) may reflect the disturbance of the balance between the functionally antagonistic α2AR and α1AR systems rather than provide insights concerning the role of the α2AAR subtype. Consequently, we manipulated the mouse genome to provide definitive evidence regarding the role of the α2AAR subtype in CNS responses.
We used the “hit and run” targeting variant of homologous recombination (6, 7) to substitute a subtle mutation of the α2aAR, D79N into the mouse genome as a tool to explore the role of the α2aAR in vivo (8). The aspartate residue at position 79 (D79) is highly conserved in a topologically identical position in the second transmembrane span in a large subset of G protein-coupled receptors (9). Mutation of this residue has been shown to eliminate allosteric regulation of receptor binding by monovalent cations (10–13) and to perturb receptor–G protein–effector coupling (14–17) in heterologous expression systems. Thus, the animals expressing the D79N α2aAR provide the opportunity to examine the functional importance of the α2AAR in a variety of complex physiological and behavioral responses.
EXPERIMENTAL PROCEDURES
Mouse Lines.
The D79N mouse line was created using a hit and run gene targeting strategy (18), as described (8). Male chimeras were mated with C57BL/6 mice to generate heterozygous mice for intercrosses. B6,129 hybrid offspring of wild-type (WT) and D79N breeding pairs were used in the present studies. Male chimeras also were mated with 129/Sv females to establish the D79N mutation on a pure 129/Sv background. These 129/Sv D79N mice showed binding properties indistinguishable from those in the mixed genetic background (other functions not evaluated). In addition, the B6,129 heterozygous offspring of a chimera have been backcrossed against C57BL/6 to establish the D79N mutation on a pure C57BL/6 background (10 generations), and the mice have been made available to Jackson Laboratories (designated Adra2atm1Lel; #2–777). The purebred C57BL/6 animals showed changes in receptor binding and in vivo sedative response indistinguishable from those on the mixed genetic background.
Rotarod and Loss of Righting Reflex Tests.
B6,129 male mice, ≈3 months old, were placed on a rotarod (IITC, Inc., Life Sciences, St. Petersburg, FL) turning at 10 revolutions per minute. The mice learned to remain on the rod during three 60-s training periods. Saline or increasing doses of dexmedetomidine (dex) were injected i.p.; after 10 min, each mouse was tested three times in succession for its ability to remain on the rod. The cutoff time was 60 s. After cumulative doses of dex (433 μg/kg) or pentobarbital (143 mg/kg) during the rotarod test, the mice were gently rolled onto their backs; mice that failed to right themselves were considered nonresponsive. Sleep time was defined as the duration of loss of righting reflex (LORR). Researchers were blinded to the genotypes of the mice tested for this and subsequent functional tests.
Anesthetic-Sparing Effect.
Groups of eight B6,129 male mice, ≈3 months old, were placed in an air-tight Plexiglas chamber with gloved access ports. Halothane (vol/vol % in O2) was continuously introduced, circulated, and monitored. Mice were equilibrated to each concentration of halothane for 40 min before testing for nonresponsiveness by LORR. After halothane concentrations that elicited LORR in the absence of exogenous drugs were established, drugs were injected i.p. and mice were tested for LORR after 30 min at the initial halothane concentration and 40 min after each change in halothane concentration.
Hot Plate Test.
B6,129 male mice, ≈3 months old, were placed on an enclosed hot-plate (IITC Inc.); the temperature of the plate was ramped at 6°C/min from 43°C to 52°C. When a mouse licked a hind paw, the mouse was removed from the hot plate and the temperature recorded. Each mouse was tested 10 min after i.p. saline or drug injections three times in succession.
NE Turnover.
B6,129 male mice, ≈3 months old, were injected i.p. with saline or dex (100 μg/kg). After 30 min, the mice were killed by 30-s exposure to CO2, and the left hippocampus was isolated. Samples were sonicated in ice-cold 5% perchloric acid and centrifuged. The supernatant was filtered to exclude molecules exceeding 5000 kDa. The biogenic amines were assayed using HPLC/electrochemical detection as described (19).
Recording of Locus Ceruleus (LC) Neuronal Activity.
Coronal brain slices of 200 μm thickness containing the LC (20) were prepared from B6,129 mice ≈2–3 weeks old using techniques described (21). Spontaneous action potentials, observed in LC neurons using the perforated patch technique, were amplified using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA) and were recorded using the pclamp program (Axon Instruments) on a computer and on a Grass Instruments (Quincy, MA) chart recorder and PCM/VCR. Drugs were delivered in the superfusing artificial cerebrospinal fluid containing 124 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 26 mM NaHCO3, 1.2 mM NaH2PO4, 10 mM d-glucose, and 2 mM CaCl2. To obtain the perforated-patch configuration, amphotericin B (200 μg/ml) was added to the internal solution, which contained 125 mM KMeSO4, 15 mM KCl, 1 mM MgCl2, 10 mM Hepes (pH 7.4), and 280 milliosmol final osmality. The firing rate and resting membrane potential of LC neurons generally were stable during the course of the experiments, which lasted 2–3 h.
Recording of Voltage-Gated Ca2+ Current.
Acutely dissociated LC neurons from B6,129 mice ≈2–3 weeks old were prepared using enzymatic treatment (21). No observable difference in morphology (22) was noticed between LC regions and isolated neurons of WT and mutant D79N mice. The neurons were superfused with external solution containing 150 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, 10 mM d-glucose (pH 7.4), 340 milliosmol final osmality. After obtaining the whole cell patch-clamp configuration, the external solution was switched to a barium solution: 140 mM tetraethylammonium (TEA)–OH, 10 mM Hepes, 5 mM BaCl2, 15 mM d-glucose, and 0.5 μM tetrodotoxin, pH 7.4) for current isolation. The current was elicited by delivering depolarizing pulses from holding potential −80 mV using an Axoclamp 1D amplifier and the pclamp program. Drugs were delivered near the neuron using an array of capillary pipes. The internal solution contained 125 mM N-methyl-d-glucamine, 20 mM TEA–OH, 10 mM Hepes, 11 mM EGTA, 1 mM CaCl2, 14 mM phosphocreatine, 4 mM Mg-ATP, 0.3 mM GTP, 10 mM HCl, pH 7.4. Superior cervical ganglion (SCG) neurons were isolated via enzymatic treatment (23), and the current was recorded as for LC neurons.
Statistical Analysis.
Group means were statistically compared using ANOVA, followed by the Tukey–Kramer multiple comparisons post hoc test, unless noted otherwise.
RESULTS
We evaluated the ability of the α2AR agonist dex to elicit sedation in D79N α2AAR mice by examining its effect on the ability of these mice to remain on a rotating bar. Dex dose-dependently reduced the ability of WT mice to remain on the rotarod (Fig. 1A), presumably by impairing motor coordination as a result of its sedative actions. Administration of the maximum dose of dex also elicited prolonged sleep time in WT mice (322 ± 38 min) whereas dex neither reduced rotarod latency nor elicited sleep in D79N mice (Fig. 1 A and B) even though dex bioavailability was similar in WT and D79N mice (plasma levels 30 min after injection: WT, 5.6 ± 4.5 ng/ml; D79N, 7.6 ± 2.6 ng/ml; n = 5–7). In contrast to the findings for the α2AR agonist dex, the non-α2AR-directed sedative agent, pentobarbital, induced comparable sleep times in WT and mutant mice (Fig. 1B). Taken together, these findings suggest that the α2AAR subtype plays a critical role in mediating the sedative-hypnotic effects of adrenergic agonists.
Figure 1.
Sedative/hypnotic and anesthetic-sparing responses in WT and D79N mice. (A) Action of dex in the rotarod test. WT (triangles) or D79N (circles) mice were administered increasing doses of dex and were placed on a rotating bar. The time that the mice were able to remain on the bar was defined as rotarod latency. Drug-naive WT or D79N mice had comparable rotarod latency. Data in this and subsequent figures are presented as mean ± SEM; n = 8 mice/group. (B) Total sleep time after drug administration. The duration of LORR was observed after dex (hatched bar) or pentobarbital (pento; open bar) administration. ∗, significantly different responses to dex in WT and D79N mice (P < 0.001, unpaired Student’s t test). (C) Concentrations of halothane required to cause LORR in the absence (squares) or presence (circles, triangles) of dex in WT and D79N mice; n = 8 mice/group. Notice that there is no leftward shift of the halothane curve in the presence of dex in D79N mice. (D) Concentrations of halothane required to cause LORR in the absence (squares) or presence (circles, triangles) of R-(2-phenylisopropyl)adenosine in WT and D79N mice; n = 8 mice/group.
A clinically useful action of α2AR agonists is their ability to reduce dose requirements for other anesthetic agents (24, 25). We evaluated the ability of dex to reduce the dose requirements for a volatile agent, halothane. In WT mice, doses of dex that were only minimally sedative in the rotarod test reduced the concentration of halothane required to elicit an anesthetic response (Fig. 1C). In contrast, dex did not elicit halothane-sparing activity in D79N α2AAR mice (Fig. 1C). The anesthetic-sparing effects of the adenosine A1-receptor (A1adoR) agonist R-(2-phenylisopropyl)adenosine were indistinguishable in WT and D79N mice (Fig. 1D), indicating that postreceptor signaling components, presumed to be common for α2AR and A1adoR (26), are not perturbed in the mutant mice. These data provide strong evidence that the dysfunction in D79N α2AAR is not caused by secondary changes in signaling pathways and, further, that the α2AAR subtype plays the prominent role in volatile anesthetic-sparing responses.
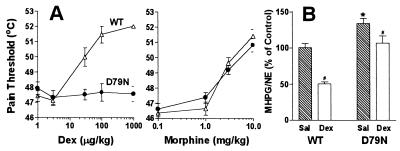
The analgesic properties of α2AR agonists add to their usefulness in the practice of anesthesiology (2, 27, 28). We examined the analgesic properties of α2AR agonists in attenuating thermally induced pain. Dex dose-dependently increased the thermal pain threshold of WT mice in the ramped hot plate test, which examines supraspinal pain perception, but had no effect on the pain threshold of D79N mice (Fig. 2A). In contrast, the μ-opioid receptor agonist morphine, a widely used analgesic agent coupled to signaling pathways similar to the α2AR, increased the pain threshold of both WT and D79N mice with indistinguishable dose-response curves (Fig. 2A). These findings indicate that the pathways mediating antinociception after thermally induced pain are not altered in the D79N α2AAR mutant mice but that the mutation of the α2AAR has eliminated responses to α2AR agonists.
Figure 2.
Analgesic effects and norepinephrine turnover in WT and D79N mice. (A) Increasing doses of dex or morphine were administered before determining the thermal pain threshold in WT (triangles) and D79N (circles) mice during the ramped hot plate test; cutoff temperature for the hot plate was 52°C. Drug doses were cumulative; n = 8 mice/group. (B) NE turnover in the hippocampus was quantitated as the ratio of the major NE metabolite MHPG to NE after injection of saline (sal) or dex in WT or D79N mice; n = 8 mice/group. ∗, significantly different WT vs D79N saline values (P < 0.01); #, significant difference between dex vs saline values for both groups (WT, P < 0.01; D79N, P < 0.05). Control turnover was defined as MHPG/NE ratio in the hippocampus in the absence of any injection.
To determine whether presynaptic effects of α2AR were modified in vivo, basal turnover of NE was measured as the ratio of the NE metabolite 3-methoxy-4-hydroxyphenylethylene glycol (MHPG) to NE, where MHPG reveals the fraction of neurotransmitter that has been released and metabolized in synaptic terminals after reuptake. The significantly elevated MHPG/NE ratio in mutant mice (Fig. 2B) suggests that regulation of presynaptic events in the CNS has been modified in the D79N α2AAR mutant mice and that the α2AAR subtype contributes to mediation of presynaptic effects, corroborating the findings of Starke and colleagues (29, 30). The retention of the ability of dex, although attenuated, to suppress the MHPG/NE ratio in the D79N mutant mice is consistent with the interpretation that the α2AAR subtype is not exclusively involved in presynaptic regulation of NE release. Our studies, however, cannot ascertain whether or not the elimination of anesthetic and analgesic responses to α2AR agonists we detect in the D79N mice are due to pre- or postsynaptic mechanisms, or both.
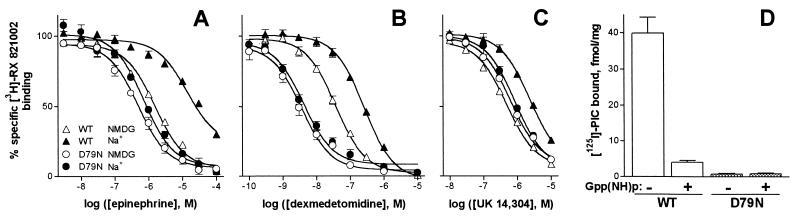
An unexpected finding was that mice homozygous for the D79N mutation exhibited a significant reduction (80%) in the functional density of α2AAR compared with the density of α2AAR in brain membranes from WT mice (8). In contrast, the content of mRNA encoding the α2AAR subtype is indistinguishable in WT and D79N mice (8) as is the temporal and spatial expression of the mRNA encoding all three α2AR subtypes (ref. 31 and R.-X. Wang and L.E.L., unpublished observations). Despite the reduction in functional α2AAR density, the specificity of binding characteristic of the α2AAR subtype was retained in the D79N mutant structure (Fig. 3A-C). The loss of response to α2AR agonists in D79N mice is not due to a reduced affinity of the D79N receptor for agonist ligands. In fact, because the D79N α2AAR lacks Na+-elicited allosteric reduction in receptor affinity for agonists, the D79N receptor actually manifests an apparent increase in affinity for agonists, the extent of which correlates with agonist efficacy (efficiency of coupling agonist occupancy to response). Thus, the increase in agonist potency is greater for dex (Fig. 3B) than for epinephrine (Fig. 3A), and there is little effect on the affinity of the D79N receptor for the agonist/partial agonist UK 14,304 (Fig. 3C) when compared with agonist competition at the WT α2AAR. The fraction of functional α2AAR that remains in D79N mice does not appear to be coupled to G proteins, at least based on guanine nucleotide regulation of agonist binding (Fig. 3D). No high affinity radiolabeled, guanine nucleotide-sensitive agonist binding was observed in D79N mice, even though we would have been able to detect 20% of the content of p-[125I]iodoclonidine binding observed in WT mice, if the only change in α2AAR binding properties in D79N mice was an 80% reduction in binding capacity. Taken together, these findings indicate that the receptor properties are those expected for the D79N mutant α2AAR (11, 32), with an unexpected reduction in the density of D79N α2AAR in vivo (8).
Figure 3.
Characterization of D79N α2AAR-binding in mouse brain. (A–C) Competition of varying agonists for [3H]RX821002 antagonist binding (8, 11) was evaluated in the absence (open symbols) or presence (closed symbols) of 100 mM Na+. Total specific binding for each condition was defined as 100%; n = 3. The affinity of [3H]RX821002 was indistinguishable in WT and mutant mice (Kd = 2.3 nM in both preparations). For these and Gpp(NH)p experiments (D), 1 μM prazosin was included in incubations to block contributions from α2BAR and α2CAR. NMDG, N-methyl-d-glucamine. (D) Specific binding of the agonist p-[125I]iodoclonidine was evaluated in the absence and presence of 100 μM Gpp(NH)p. Notice that the binding to D79N α2AAR is minimal even in the absence of Gpp(NH)p. Gpp(NH)p, 5′-guanylyl β,γ-imidodiphosphate.
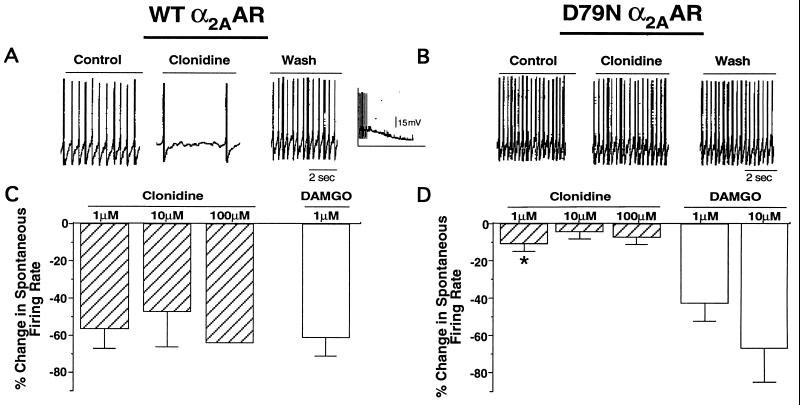
We evaluated D79N α2AAR regulation of ion channels in LC neurons. The LC is the site for expression of α2AR-mediated, sedative-hypnotic responses (33, 34) and participates in α2AR-induced analgesic effect (35, 36). In situ hybridization in the mouse brain has revealed dense α2AAR localization in the LC (31), thus facilitating electrical detection of α2AAR-expressing neurons. LC neurons fire spontaneous action potentials. Activation of α2AAR or μ-opioid receptors in the LC enhances outward K+ current through inwardly rectifying K+ channels, leading to neuronal hyperpolarization and consequent reduction in spontaneous firing frequency (37–39). As shown in Fig. 4 A and C, the α2AR agonist clonidine elicited a significant suppression of spontaneous firing rate recorded from LC neurons in the brain slice preparation from WT mice, which was accompanied by detectable hyperpolarization in some neurons (Fig. 4A Inset). In contrast, treatment of LC neurons from D79N mice failed to alter spontaneous firing rate or membrane potential, even at supramaximal concentrations of the α2AR agonist (Fig. 4 B and D). (Clonidine, rather than dex, was used in these studies because its effects on brain slice preparations were more rapidly reversible.) The lack of response to exogenous agonist in D79N neurons was not due to a tonically active receptor population because α2AR antagonists failed to influence spontaneous firing in mutant preparations (data not shown). In fact, the basal firing rate was similar in neurons expressing either WT or D79N α2AAR (WT, 3.09 ± 0.34 Hz; D79N, 2.96 ± 0.41 Hz; n = 10–23). Furthermore, the μ-opioid receptor agonist [d-Ala2, N-McPhe4, Gly5-ol]enkephalin (DAMGO) elicited comparable inhibition of firing in WT and mutant neurons (Fig. 4 C and D). The attenuation of spontaneous firing in the LC is attributed primarily to activation of inwardly rectifying K+ channels (37–39), so the observation that the D79N α2AAR is unable to regulate LC neuronal firing suggests that the α2AAR cannot activate these K+ currents in D79N mice.
Figure 4.
Modulation of spontaneous firing rate in WT and D79N LC neurons in the brain slice preparation. (A and B) Representative traces depicting spontaneous firing under control conditions in the presence of clonidine (1 μM) and after wash of clonidine. (Inset) Clonidine-induced hyperpolarization in a WT α2AAR-expressing LC neuron. Clonidine was administered at an earlier time point not shown in the trace. (C and D) Percentage inhibition of spontaneous firing rate by clonidine and DAMGO in WT and D79N LC neurons. The neurons were obtained from 2–11 animals per each treatment. The numbers of neurons tested were: clonidine (1 μM) 8–15; clonidine (10 μM) 3–4; clonidine (100 μM) 2–3; DAMGO (1 μM) 10–17; and DAMGO (10 μM) 5. ∗, significantly different WT vs D79N value for the same treatment (P < 0.05). Clonidine (10 and 100 μM) groups were not included in the statistical analysis because of low sample size.
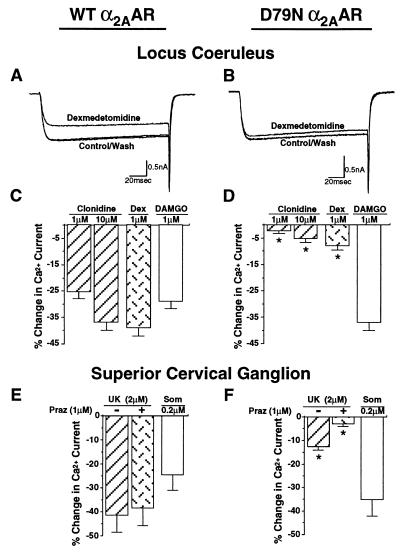
Activation of α2AR results in inhibition of voltage-gated Ca2+ channels in many neuronal preparations. Suppression of these currents in LC neurons has been implicated in sedative-hypnotic actions of α2AR agonists (34). A similar mechanism may underlie α2AR-mediated anesthetic-sparing effects (40, 41). Evaluation of α2AR attenuation of voltage-gated Ca2+ currents in WT and mutant mice was performed in acutely dissociated LC neurons to permit better voltage clamp control than obtainable in brain slices. Stimulation of α2AAR with both clonidine and dex significantly attenuated voltage-gated Ca2+ current in LC neurons isolated from WT mice (Fig. 5 A and C); this response was dramatically reduced, however, in LC neurons derived from D79N mutant mice (Fig. 5 B and D). The difference between α2AAR responses in WT and mutant neurons could not be attributed to any fundamental change in Ca2+ current because the current–voltage relationship for Ca2+ current was indistinguishable in both neuronal preparations (data not shown). In addition, DAMGO-mediated inhibition of Ca2+ currents was similar in both WT and mutant neurons (Fig. 5 C and D), indicating that the loss of regulation of Ca2+ current by D79N α2AAR reflects a dysfunction of the mutant receptor rather than a fundamental change in the neuronal population or its regulation by other Gi/Go-coupled receptors. The lack of effect of α2AR antagonists on the Ca2+ currents measured in either the WT or D79N preparations in the absence of agonist suggests that the responses noted are not influenced by the presence of endogenous agonist.
Figure 5.
Inhibition of voltage-gated Ca2+ current in acutely dissociated WT and D79N LC and SCG neurons. (A and B) Representative traces depicting the Ca2+ currents, generated by a pulse from −80 to −10 mV, in the absence and presence of dex (1 μM) in WT and D79N LC neurons. The currents were leak-subtracted on-line. The amplitude of the Ca2+ current ranged from −0.2 to −3nA in various LC neurons and was not significantly different in WT and mutant receptor-expressing neurons. (C and D) Percentage inhibition of the Ca2+ current by clonidine, dex, and DAMGO in WT and mutant LC neurons. Neurons from at least three mice were used for each treatment. The number of neurons tested were: clonidine (1 μM), 8–10; clonidine (10 μM), 8–16; dex, 13; DAMGO, 10–16. ∗, significantly different WT vs D79N value for the same treatment (P < 0.001). (E and F) Percentage inhibition of the Ca2+ current in WT and D79N SCG neurons by UK 14,304 in the absence and presence of the α1AR, α2BAR, and α2CAR antagonist prazosin and by somatostatin. The numbers of neurons tested were: UK 14,304, 7–14; UK 14,304 plus prazosin, 3–7; and Som, 3–8. ∗, significantly different WT vs D79N value for the same treatment (P < 0.01). Notice that >80% of the UK 14,304-mediated response in WT is insensitive to 1 μM prazosin, consistent with an α2AAR response. In contrast, much of the residual UK 14,304 suppression of Ca2+ current in D79N mice is prazosin-sensitive, perhaps reflective of α2B or α2CAR mediation of UK 14,304 effects in this setting.
We also examined α2AR-elicited suppression of voltage-gated Ca2+ current in SCG neurons. Stimulation of WT α2AR with the agonists UK 14,304 (Fig. 5E), clonidine, and dex (data not shown) produced significant inhibition of Ca2+ currents in WT SCG neurons. The considerably attenuated α2AR-agonist-induced inhibition of Ca2+ currents in SCG neurons from mutant mice was completely suppressed by prazosin (Fig. 5F), an α1AR antagonist that also blocks α2BAR and α2CAR subtypes (but not the α2AAR subtype) with reasonably high potency (5), suggesting that the residual voltage-gated Ca2+ current response to α2AR agonists in neurons from D79N mice is mediated by α2B and/or α2CAR subtype. Thus, the D79N mutant α2AAR is unable to evoke inhibition of voltage-gated Ca2+ currents in SCG neurons, similar to the loss of responses observed in LC neurons derived from D79N mutant mice. In contrast, the somatostatin receptor, another Gi/Go-coupled receptor, evoked a similar degree of Ca2+ current suppression in both WT and mutant SCG neuronal preparations (Fig. 5 E and F), again indicating that the D79N mutation results in loss of α2AAR response without perturbation of shared downstream G protein or effector pathways.
DISCUSSION
The present study provides definitive evidence that the α2A subtype of α2AR mediates clinically relevant sedative-hypnotic, analgesic, and anesthetic-sparing responses in the mouse. In the absence of subtype-specific agonists and antagonists for the three α2AR subtypes (5), manipulation of the mouse genome provides an alternative and, at present, the sole approach for defining the role of particular receptor subtypes in in vivo responses. An earlier study implicating the α2aAR subtype in sedative responses used antisense strategies to attenuate α2AAR vs α2CAR expression; however, the antisense oligonucleotides directed against the α2aAR subtype only reduced, but did not abolish, the sedative response, and thus the role of another α2AR subtype in the residual response could not be precluded (42). In contrast, the data from D79N α2AAR mice established the α2AAR subtype as the predominant, and likely the only, subtype mediating the sedative-hypnotic response.
Involvement of the α2AAR subtype in the analgesic response has been suggested on the basis of a comparison of the efficacy of nonsubtype-specific α2AR agents in producing the response in vivo and the affinity of these agents in interacting with the receptor in vitro (43). However, variability in distribution and bioavailability of α2AR agents could not be controlled in this study and may have confounded the interpretations, a limitation acknowledged by the authors (43). In comparison, the present studies, which exploit a genetic approach, have unequivocally demonstrated that the α2AAR subtype mediates analgesic effects of α2AR agonists in attenuating thermal pain perception. The complete loss of response to α2AR agonists in sedative and analgesic paradigms indicates that the α2B and/or α2CAR do not contribute to these responses; otherwise, they would have been able to compensate for the D79N α2AAR mutation.
It is of interest that a single α2AR subtype is pivotal for both sedative and analgesic effects, especially because the proposed sites for these actions of α2AR differ significantly. The LC has been implicated in the sedative effect of α2AR agonists (33), and the analgesic response to α2AR agonists occurs via both supraspinal (including the LC) and spinal loci (36, 44, 45). Our studies indicate that mutation of the α2AAR eliminates sedative-hypnotic as well as analgesic responses to α2AR agonists without causing a general perturbation of the neural system because other non-α2AR-directed agents produce expected physiological and electrophysiological responses.
The findings from electrophysiological studies provide direct evidence that the D79N α2AAR is unable to activate inwardly rectifying K+ channels and to inhibit voltage-gated Ca2+ channels. Whether the loss of D79N α2AAR modulation of these effector systems in vivo is due to a perturbation of receptor-G protein coupling by the mutation, as observed in these studies (cf. Fig. 3D) as well as under in vitro conditions (14), or is due to the ≈80% reduction in D79N α2AAR binding sites in the mutant mice without alteration in the quantity of mRNA encoding the WT vs D79N α2AAR (8) is not known. It is likely that the selective uncoupling of the D79N α2AAR to K+ channels observed in AtT20 cells, compared at equivalent WT and mutant α2AAR density (16, 17), was not observed here because of the unexpected but marked reduction in the D79N α2AAR functional binding capacity in vivo. However, because physiological responses in vivo differ in their requirement for α2AR occupancy (46), the reduction in α2AAR density in mutant mice will affect physiological responses differently, making it difficult to determine the relative contribution of receptor density vs altered receptor-G protein coupling to the loss of various responses in the D79N α2AAR mutant mice.
In summary, the present study provides definitive evidence that the α2AAR subtype is the primary mediator of the sedative, analgesic, and anesthetic-sparing responses to α2AR agonists and also participates in presynaptic autoinhibition of neurotransmitter release. Although we cannot assign an unequivocal molecular explanation (i.e., loss of receptor density or G protein communication or both) for the loss of each of the electrical, cellular, and physiological responses evaluated in these studies, the present functional findings indicate that the D79N α2AAR mutant mice provide a powerful tool for elucidating the role of the α2AAR subtype in complex and diverse physiological responses in vivo, an essential prelude for the design of subtype-selective therapeutic agents.
Acknowledgments
P.P.L. thanks Dr. Sukwoo Choi for teaching her the brain slice preparation and recording technique. L.B.M. is grateful to Drs. Mark Magnuson, Earl Ruley, Brigid Hogan (all of Vanderbilt University), Colin Funk (Vanderbilt University, now University of Pennsylvania), and Lutz Birnbaumer (Baylor University, now University of California Los Angeles) for advice and consultation in early stages of this study. This study was supported by the Department of Veterans Affairs (M. M. Merit Review Award), a National Alliance for Research on Schizophrenia and Depression Established Investigator Award (L.E.L.), a Vanderbilt University Graduate Fellowship (L.B.M.), and grants from the National Institutes of Health (M.M., GM30232; D.M.L., NS30470; and L.E.L., HL25182 and HL43671).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: α2AR, α2-adrenergic receptor; cDNA or genes encoding the α2AR subtypes are designated as α2aAR, α2bAR, and α2cAR; the pharmacologically defined subtype proteins are designated as α2AAR, α2BAR, and α2CAR; CNS, central nervous system; WT, wild-type; dex, dexmedetomidine; LC, locus ceruleus; LORR, loss of righting reflex; MHPG, 3-methoxy-4-hydroxyphenylethylene glycol; NE, norepinephrine; SCG, superior cervical ganglion; DAMGO, [d-Ala2, N-McPhe4, Gly5-ol]enkephalin.
References
- 1.Ruffolo R R, Jr, Nichols A J, Stadel J M, Hieble J P. Annu Rev Pharmacol Toxicol. 1993;33:243–279. doi: 10.1146/annurev.pa.33.040193.001331. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman B B, Lefkowitz R J. In: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. pp. 199–248. [Google Scholar]
- 3.Bylund D B, Eikenburg D C, Hieble J P, Langer S Z, Lefkowitz R J, Minneman K P, Molinoff P B, Ruffolo R R, Jr, Trendelenburg U. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 4.Limbird L E. FASEB J. 1988;2:2686–2695. doi: 10.1096/fasebj.2.11.2840317. [DOI] [PubMed] [Google Scholar]
- 5.Hieble J P, Ruffolo R R., Jr Prog Drug Res. 1996;47:81–130. [PubMed] [Google Scholar]
- 6.Hasty P, Ramirez-Solis R, Krumlanf R, Bradley A. Nature (London) 1991;350:243–246. doi: 10.1038/350243a0. [DOI] [PubMed] [Google Scholar]
- 7.Valancius V, Smithies O. Mol Cell Biol. 1991;11:1402–1408. doi: 10.1128/mcb.11.3.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacMillan L B, Hein L, Smith M S, Piascik M T, Limbird L E. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- 9.Probst W C, Snyder L A, Schuster D I, Brosius J, Sealfon S C. DNA Cell Biol. 1992;11:1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- 10.Tian W-N, Deth R C. Life Sci. 1993;52:1899–1907. doi: 10.1016/0024-3205(93)90630-l. [DOI] [PubMed] [Google Scholar]
- 11.Horstman D A, Brandon S, Wilson A L, Guyer C A, Cragoe E J, Jr, Limbird L E. J Biol Chem. 1990;265:21590–21595. [PubMed] [Google Scholar]
- 12.Neve K A, Cox B A, Hinningsen R A, Spanoyannis A, Neve R L. Mol Pharmacol. 1993;39:733–737. [PubMed] [Google Scholar]
- 13.Kong H, Raynor K, Yasuda K, Moe S T, Portoghese P S, Bell G, Reisine T. J Biol Chem. 1993;268:23055–23058. [PubMed] [Google Scholar]
- 14.Ceresa B P, Limbird L E. J Biol Chem. 1994;269:29557–29564. [PubMed] [Google Scholar]
- 15.Chung F Z, Wang C D, Potter P C, Venter J C, Fraser C M. J Biol Chem. 1988;263:4052–4055. [PubMed] [Google Scholar]
- 16.Surprenant A, Horstman D A, Akbarali H, Limbird L E. Science. 1992;257:977–980. doi: 10.1126/science.1354394. [DOI] [PubMed] [Google Scholar]
- 17.Lakhlani P P, Lovinger D M, Limbird L E. Mol Pharmacol. 1996;50:96–103. [PubMed] [Google Scholar]
- 18.Ramirez-Solis R, Davis A C, Bradley A. Methods Enzymol. 1993;225:855–878. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M, Zornow M H, Rabin B C, Maze M. Brain Res. 1993;627:325–329. doi: 10.1016/0006-8993(93)90337-m. [DOI] [PubMed] [Google Scholar]
- 20.Masuko S, Nakajima Y, Nakajima S, Yamaguchi K. J Neurosci. 1986;6:3229–3241. doi: 10.1523/JNEUROSCI.06-11-03229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi S, Lovinger D M. J Neurosci. 1996;16:36–45. doi: 10.1523/JNEUROSCI.16-01-00036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson L W. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda S R, Lovinger D M, McCool B A, Lewis D L. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 24.Segal I S, Vickery R G, Walton J K, Doze V A, Maze M. Anesthesiology. 1988;69:818–823. doi: 10.1097/00000542-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Vickery R G, Sheridan B C, Segal I S, Maze M. Anesth Analg. 1988;67:611–615. [PubMed] [Google Scholar]
- 26.van Galen P J M, Stiles G L, Michaels G, Jacobson K A. Med Res Rev. 1992;12:423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal I S, Javis D J, Duncan S R, White P F, Maze M. Anesthesiology. 1991;74:220–225. doi: 10.1097/00000542-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi Y, Maze M. Br J Anesthesiol. 1993;71:108–118. doi: 10.1093/bja/71.1.108. [DOI] [PubMed] [Google Scholar]
- 29.Limberger N, Trendelenburg A U, Starke K. Naunyn-Schmiedebergs Arch Pharmacol. 1995;352:43–48. doi: 10.1007/BF00169188. [DOI] [PubMed] [Google Scholar]
- 30.Trendelenburg A U, Limberger N, Starke K. J Pharmacol Exp Ther. 1996;278:462–467. [PubMed] [Google Scholar]
- 31.Wang R, MacMillan L B, Fremeau R T, Jr, Magnuson M A, Linder J, Limbird L E. Neuroscience. 1996;74:199–218. doi: 10.1016/0306-4522(96)00116-9. [DOI] [PubMed] [Google Scholar]
- 32.Chabre O, Conklin B R, Brandon S, Bourne H R, Limbird L E. J Biol Chem. 1994;269:5730–5734. [PubMed] [Google Scholar]
- 33.Correa-Sales C, Rabin B C, Maze M. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Nacif-Coelho C, Correa-Sales C, Chang L L, Maze M. Anesthesiology. 1994;81:1527–1534. doi: 10.1097/00000542-199412000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Margalit D, Segal M. Psychopharmacology. 1979;62:169–173. doi: 10.1007/BF00427132. [DOI] [PubMed] [Google Scholar]
- 36.Guo T-Z, Jiang J Y, Buttermann A E, Maze M. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Aghajanian G K, VanderMaelen C P. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- 38.Nestler E J, Alreja M, Aghajanian G K. Brain Res Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 39.Williams J T, Henderson G, North R A. Neuroscience. 1985;14:95–101. doi: 10.1016/0306-4522(85)90166-6. [DOI] [PubMed] [Google Scholar]
- 40.Dolin S J, Little H J. Br J Pharmacol. 1986;88:904–914. doi: 10.1111/j.1476-5381.1986.tb16265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maze M. Int Anesthesiol Clin. 1989;27:248–258. doi: 10.1097/00004311-198902740-00003. [DOI] [PubMed] [Google Scholar]
- 42.Mizobe T, Maghsoudi K, Sitwala K, Tianzhi G, Ou J, Maze M. J Clin Invest. 1996;98:1076–1080. doi: 10.1172/JCI118887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millan M J, Bervoets K, Rivet J-M, Widdowson P, Renouard A, Le Marouille-Girardon, Gobert A. J Pharmacol Exp Ther. 1994;270:958–972. [PubMed] [Google Scholar]
- 44.Yaksh T L. Pharmacol Biochem Behav. 1985;22:845–858. doi: 10.1016/0091-3057(85)90537-4. [DOI] [PubMed] [Google Scholar]
- 45.Pertovaara A, Kauppila T, Jyvasjarvi E, Kalso E. Neuroscience. 1991;44:705–714. doi: 10.1016/0306-4522(91)90089-7. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi Y, Guo T-Z, Maze M. Anesthesiology. 1995;82:954–962. doi: 10.1097/00000542-199504000-00019. [DOI] [PubMed] [Google Scholar]