Abstract
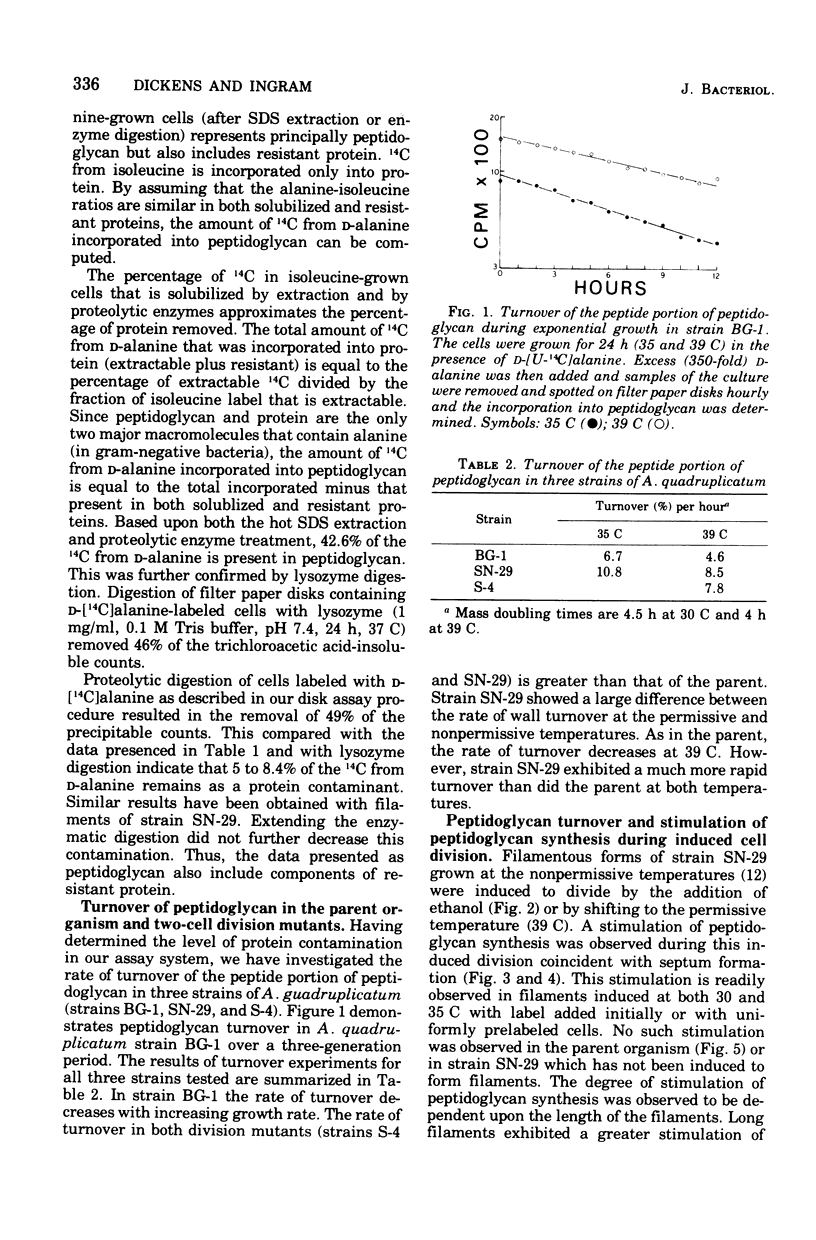
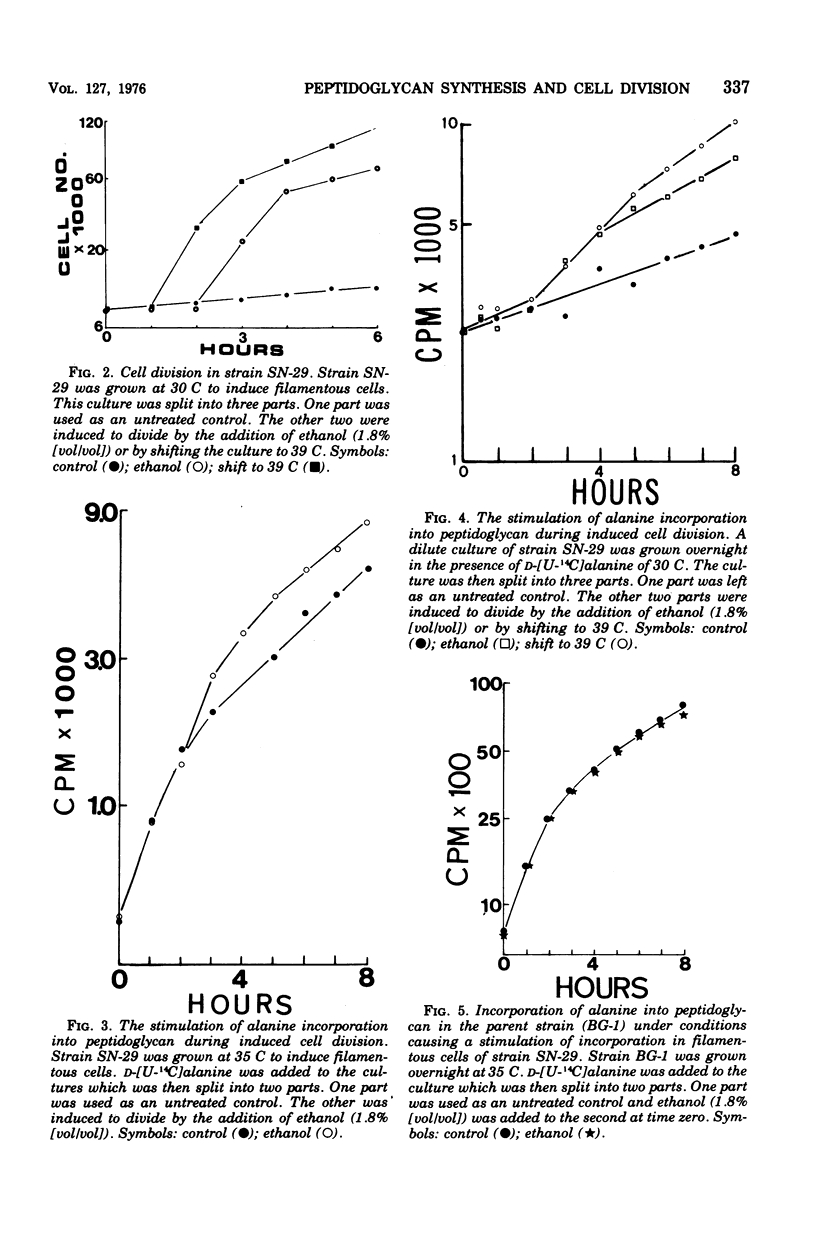
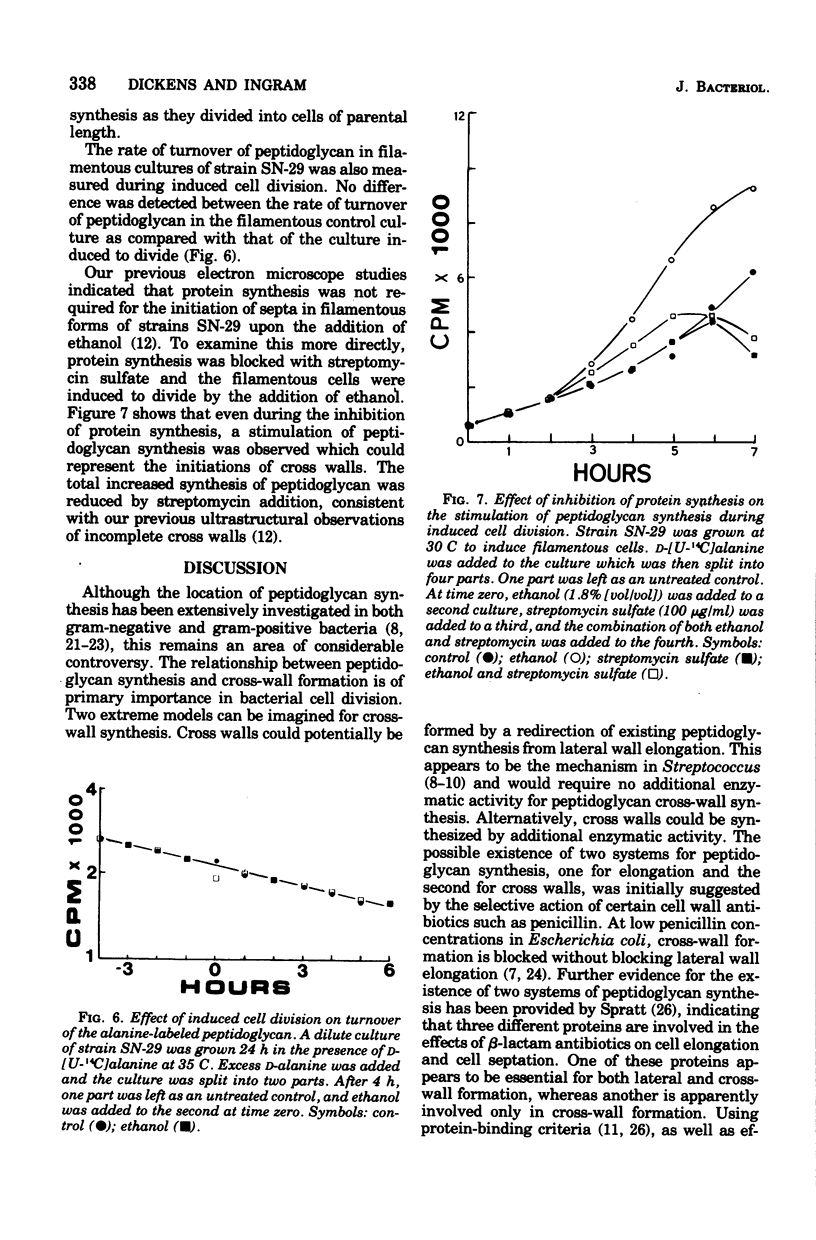
The synthesis and turnover of peptidoglycan in Agmenellum quadruplicatum was investigated using D-[U-14C]alanine followed by proteolytic digestion. The rate of turnover of alanine in the peptide portion of the peptidoglycan was measured in strain BG-1 and in two division mutants of this strain: one was blocked in cell separation; and the other was a low-temperature, conditional cell division mutant. The peptide portion of peptidoglycan turned over in all three strains tested, but no correlation was observed between septum formation or cell separation and the rate of turnover. Peptidoglycan synthesis was measured during induced division in snake forms of strain SN-29. A stimulation of peptidoglycan synthesis was observed during the period of cross-wall formation, even in the absence of new protein synthesis. Thus in A. quadruplicatum, cross-wall synthesis is accompanied by a stimulation of peptidoglycan synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Chaloupka J., Krecková P., Cáslavská J., Strnadová M. Turnover of murein in cellular and filamentous populations of Bacillus megaterium. Folia Microbiol (Praha) 1974;19(4):257–263. doi: 10.1007/BF02873217. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Turnover of mucopeptide during the life cycle of Bacillus megaterium. Folia Microbiol (Praha) 1971;16(5):372–382. doi: 10.1007/BF02875757. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Strnadová M. Turnover of murein in a diaminopimelic acid dependent mutant of Escherichia coli. Folia Microbiol (Praha) 1972;17(6):446–455. doi: 10.1007/BF02872729. [DOI] [PubMed] [Google Scholar]
- ECHLIN P., MORRIS I. THE RELATIONSHIP BETWEEN BLUE-GREEN ALGAE AND BACTERIA. Biol Rev Camb Philos Soc. 1965 May;40:143–187. doi: 10.1111/j.1469-185x.1965.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Glaser L. Bacterial cell surface polysaccharides. Annu Rev Biochem. 1973;42:91–112. doi: 10.1146/annurev.bi.42.070173.000515. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Reinitiation of cell wall growth after threonine starvation of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):1175–1183. doi: 10.1128/jb.105.3.1175-1183.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Fisher W. D. Mechanism for the regulation of cell division in Agmenellum. J Bacteriol. 1973 Feb;113(2):1006–1014. doi: 10.1128/jb.113.2.1006-1014.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Fisher W. D. Selective inhibition of deoxyribonucleic acid synthesis by 2-deoxyadenosine in the blue-green bacterium Agmenellum quadruplicatum. J Bacteriol. 1972 Oct;112(1):170–175. doi: 10.1128/jb.112.1.170-175.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Fisher W. D. Stimulation of cell division by membrane-active agents. Biochem Biophys Res Commun. 1973 Jan 23;50(2):200–210. doi: 10.1016/0006-291x(73)90827-9. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Van Baalen C. Characteristics of a stable, filamentous mutant of a coccoid blue-green alga. J Bacteriol. 1970 Jun;102(3):784–789. doi: 10.1128/jb.102.3.784-789.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Van Baalen C., Fisher W. D. Cell division mutations in the blue-green bacterium Agmenellum quadruplicatum strain BG1: a comparison of the cell wall. J Bacteriol. 1972 Aug;111(2):614–621. doi: 10.1128/jb.111.2.614-621.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. Identification of an outer membrane protein of Escherichia coli, with a role in the coordination of deoxyribonucleic acid replication and cell elongation. J Bacteriol. 1975 Nov;124(2):918–929. doi: 10.1128/jb.124.2.918-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Glaser L. Turnover of the cell wall of Bacillus subtilis W-23 during logarithmic growth. Biochem Biophys Res Commun. 1970 May 22;39(4):699–706. doi: 10.1016/0006-291x(70)90261-5. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J Mol Biol. 1975 Nov 15;98(4):749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon J. A., Aldrich H. A., Ingram L. O. Cell division in blue-green bacteria: stimulation of localized regions of peptidoglycan metabolism by ethanol. Microbios. 1975;12(49):143–154. [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


