Abstract
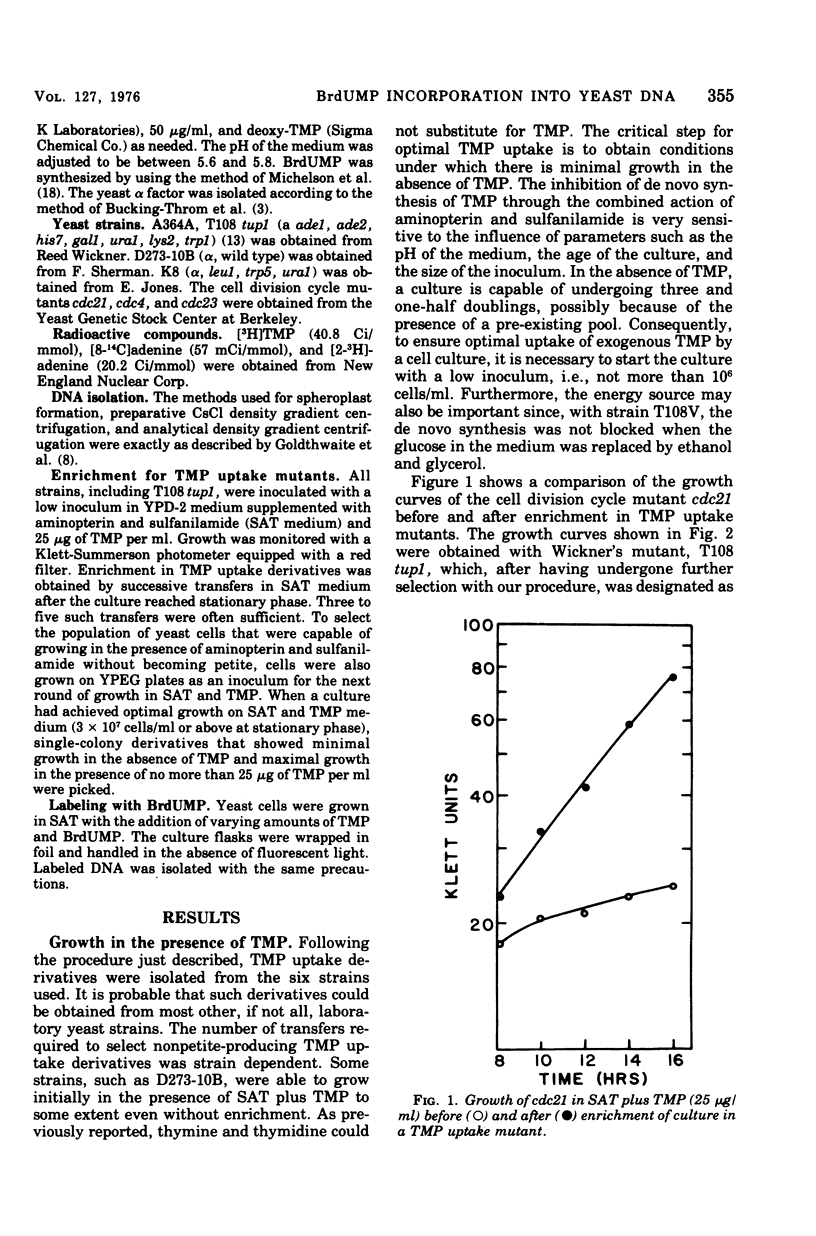
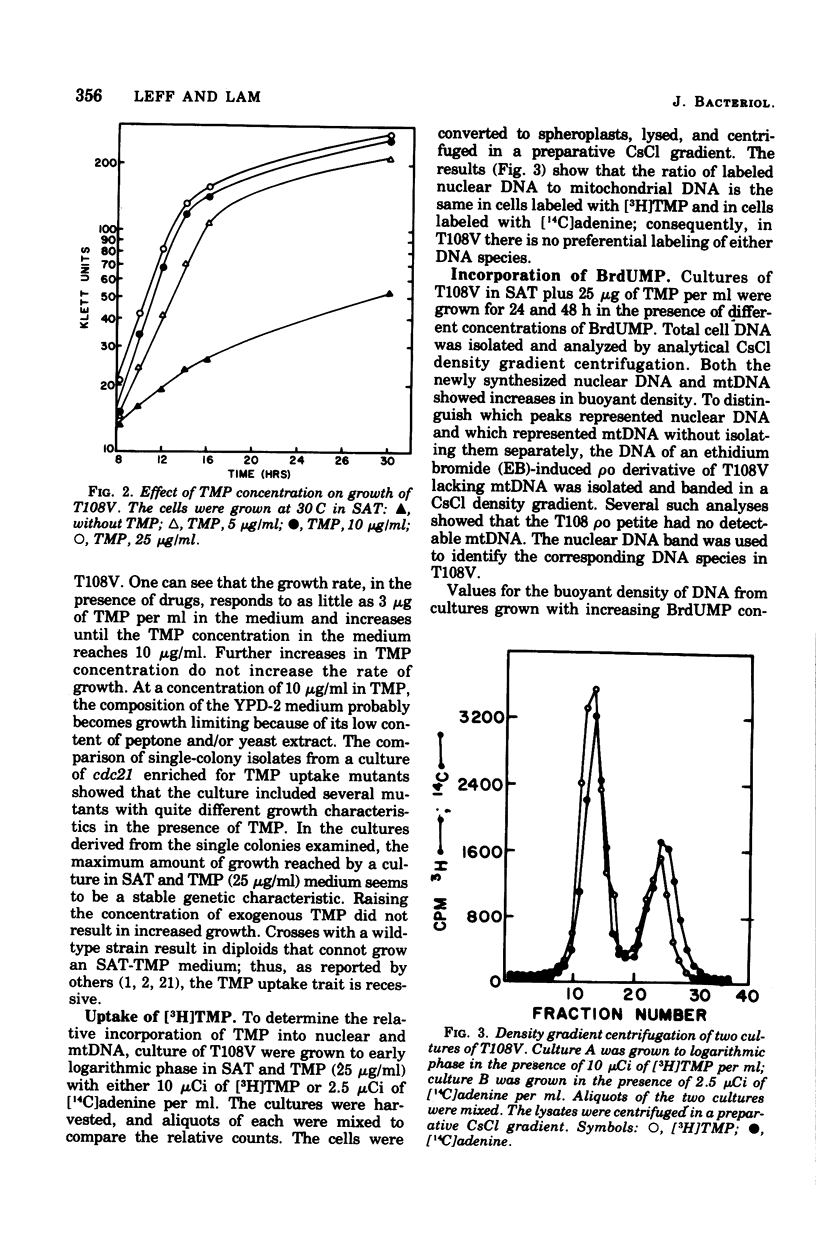
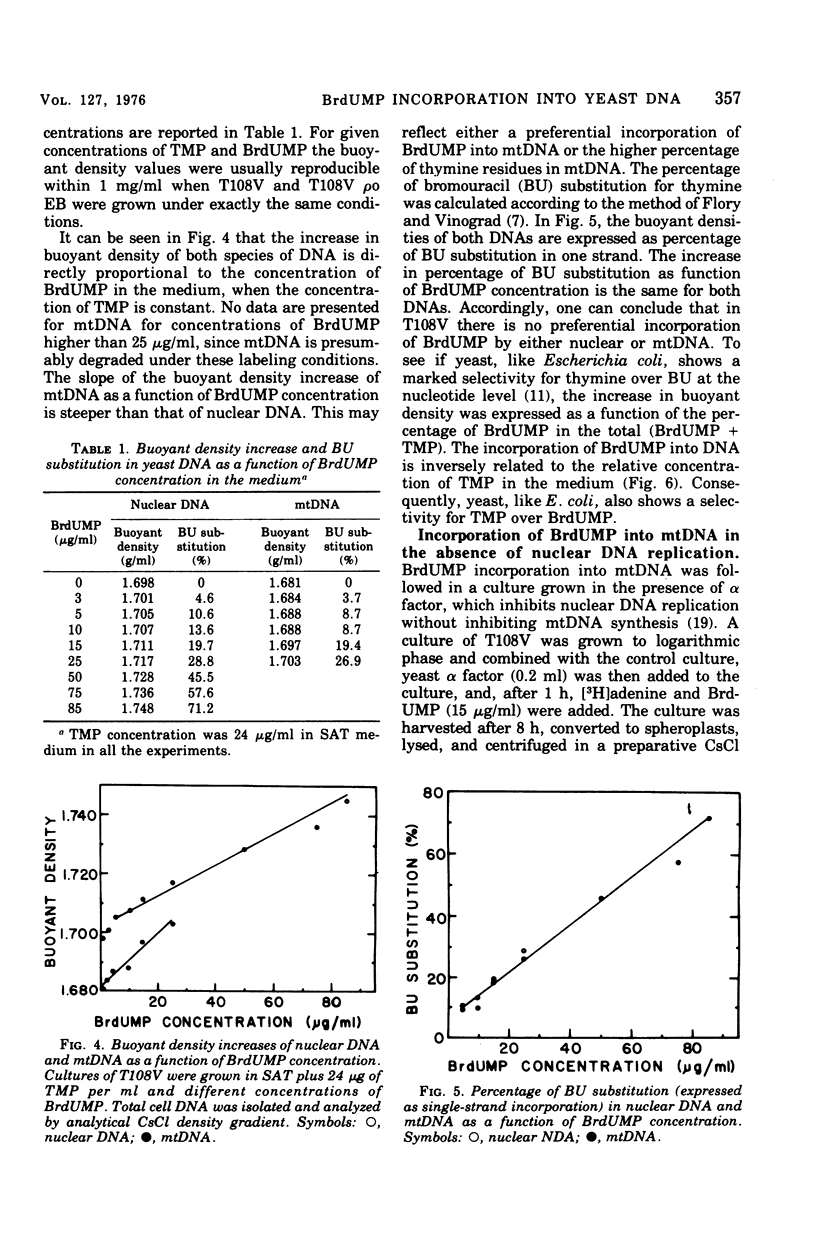
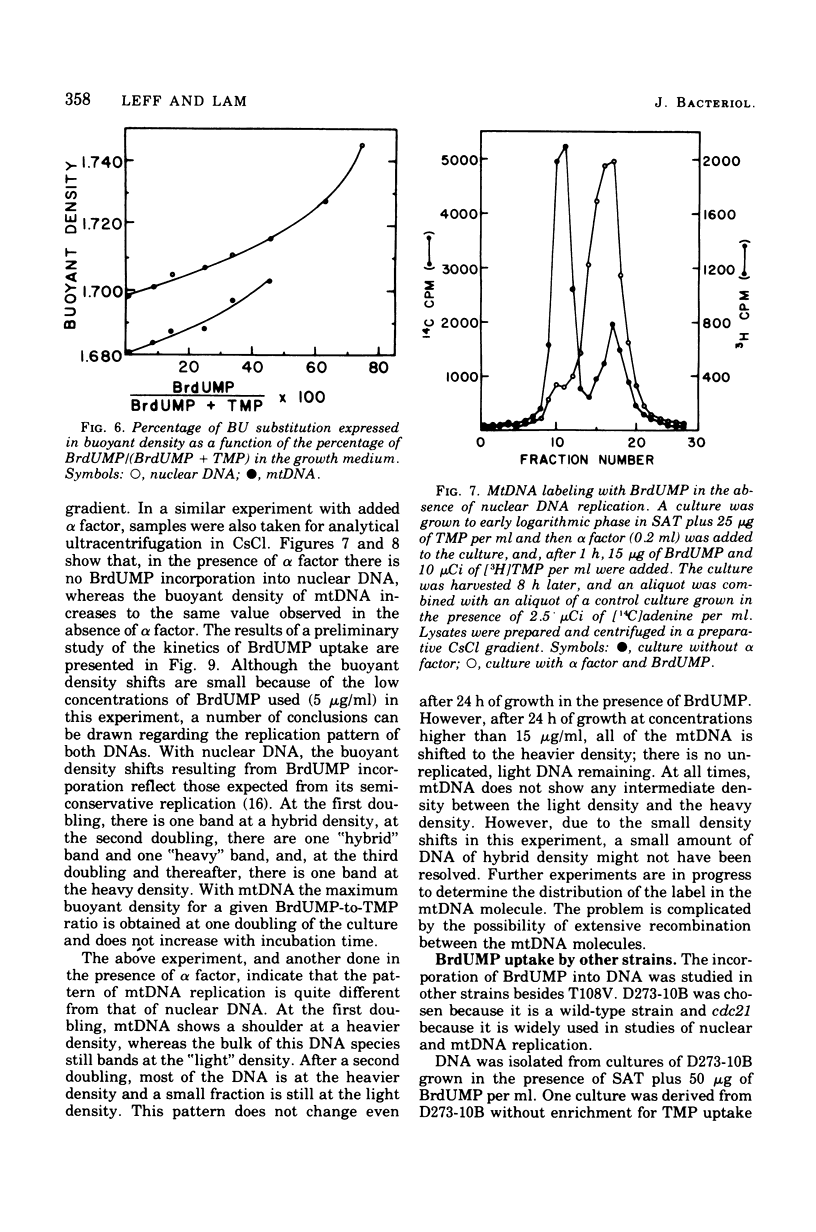
Standard laboratory yeast strains can be enriched for thymidine 5'-monophosphate (TMP) uptake derivatives that generate only a low percentage of respiratory-deficient colonies (petites) under inhibition of TMP biosynthesis. Such mutants incorporated bromodeoxyuridine 5'-monophosphate (BrdUMP) into both nuclear and mitochondrial deoxyribonucleic acid (mtDNA); however, they showed a selectivity for TMP over BrdUMP incorporation. The preferential incorporation of [3H]TMP or BrdUMP into mtDNA was strain dependent. The density increments after growth in the presence of BrdUMP reached 50 mg/ml for nuclear DNA and 22 mg/ml for mtDNA in CsCl gradients. Density shifts corresponding to 4% bromouracil substitution were easily detected. Preliminary density transfer experiments confirm that mtDNA does not replicate in synchrony with nuclear DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brendel M., Fäth W. W., Laskowski W. Isolation and characterization of mutants of Saccharomyces cerevisiae able to grow after inhibition of dTMP synthesis. Methods Cell Biol. 1975;11:287–294. doi: 10.1016/s0091-679x(08)60329-5. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Kinetics and genetic control of the incorporation of thymidine monophosphate in yeast DNA. Mol Gen Genet. 1972;117(1):39–44. doi: 10.1007/BF00268835. [DOI] [PubMed] [Google Scholar]
- Corneo G., Moore C., Sanadi D. R., Grossman L. I., Marmur J. Mitochondrial DNA in yeast and some mammalian species. Science. 1966 Feb 11;151(3711):687–689. doi: 10.1126/science.151.3711.687. [DOI] [PubMed] [Google Scholar]
- Flory P. J., Jr, Vinograd J. 5-bromodeoxyuridine labeling of monomeric and catenated circular mitochondrial DNA in HeLa cells. J Mol Biol. 1973 Feb 25;74(2):81–94. doi: 10.1016/0022-2836(73)90100-9. [DOI] [PubMed] [Google Scholar]
- Fäth W. W., Brendel M., Laskowski W., Lehmann-Brauns E. Economizing DNA-specific labelling by deoxythymidine-5'-monophosphate in Saccharomyces cerevisiae. Mol Gen Genet. 1974;132(4):335–345. doi: 10.1007/BF00268573. [DOI] [PubMed] [Google Scholar]
- Fäth W. W., Brendel M. Specific DNA-labelling by exogenous thymidine-5'-monophosphate in Saccharomyces cerevisiae. Mol Gen Genet. 1974;131(1):57–67. doi: 10.1007/BF00269387. [DOI] [PubMed] [Google Scholar]
- Goldthwaite C. D., Cryer D. R., Marmur J. Effect of carbon source on the replication and transmission of yeast mitochondrial genomes. Mol Gen Genet. 1974;133(2):87–104. doi: 10.1007/BF00264830. [DOI] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Rabinowitz M. Synthesis of new strands of mitochondrial and nuclear deoxyribonucleic acid by semiconservative replication. J Biol Chem. 1969 Mar 25;244(6):1563–1566. [PubMed] [Google Scholar]
- Hackett P., Jr, Hanawalt P. Selectivity for thymine over 5-bromouracil by a thymine-requiring bacterium. Biochim Biophys Acta. 1966 Aug 17;123(2):356–363. doi: 10.1016/0005-2787(66)90288-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Reid B. Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci U S A. 1970 Jun;66(2):352–359. doi: 10.1073/pnas.66.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson F. The lesions produced by ultraviolet light in DNA containing 5-bromouracil. Q Rev Biophys. 1973 May;6(2):201–246. doi: 10.1017/s0033583500001141. [DOI] [PubMed] [Google Scholar]
- Jannsen S., Witte I., Megnet R. Mutants for the specific labelling of DNA in Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Apr 11;299(4):681–685. doi: 10.1016/0005-2787(73)90243-8. [DOI] [PubMed] [Google Scholar]
- MICHELSON A. M., DONDON J., GRUNBERG-MANAGO M. The action of polynucleotide phosphorylase on 5-halogenouridine-5' pyrophosphates. Biochim Biophys Acta. 1962 Apr 2;55:529–540. doi: 10.1016/0006-3002(62)90986-1. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Preferential synthesis of yeast mitochondrial DNA in alpha factor-arrested cells. Biochem Biophys Res Commun. 1973 Dec 10;55(3):603–609. doi: 10.1016/0006-291x(73)91186-8. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine 5'-monophosphate into DNA in vivo. Methods Cell Biol. 1975;11:295–302. doi: 10.1016/s0091-679x(08)60330-1. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. doi: 10.1128/jb.117.1.252-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. Apparent dispersive replication of yeast mitochondrial DNA as revealed by density labelling experiments. Mol Gen Genet. 1974;131(3):193–207. doi: 10.1007/BF00267959. [DOI] [PubMed] [Google Scholar]



