Abstract
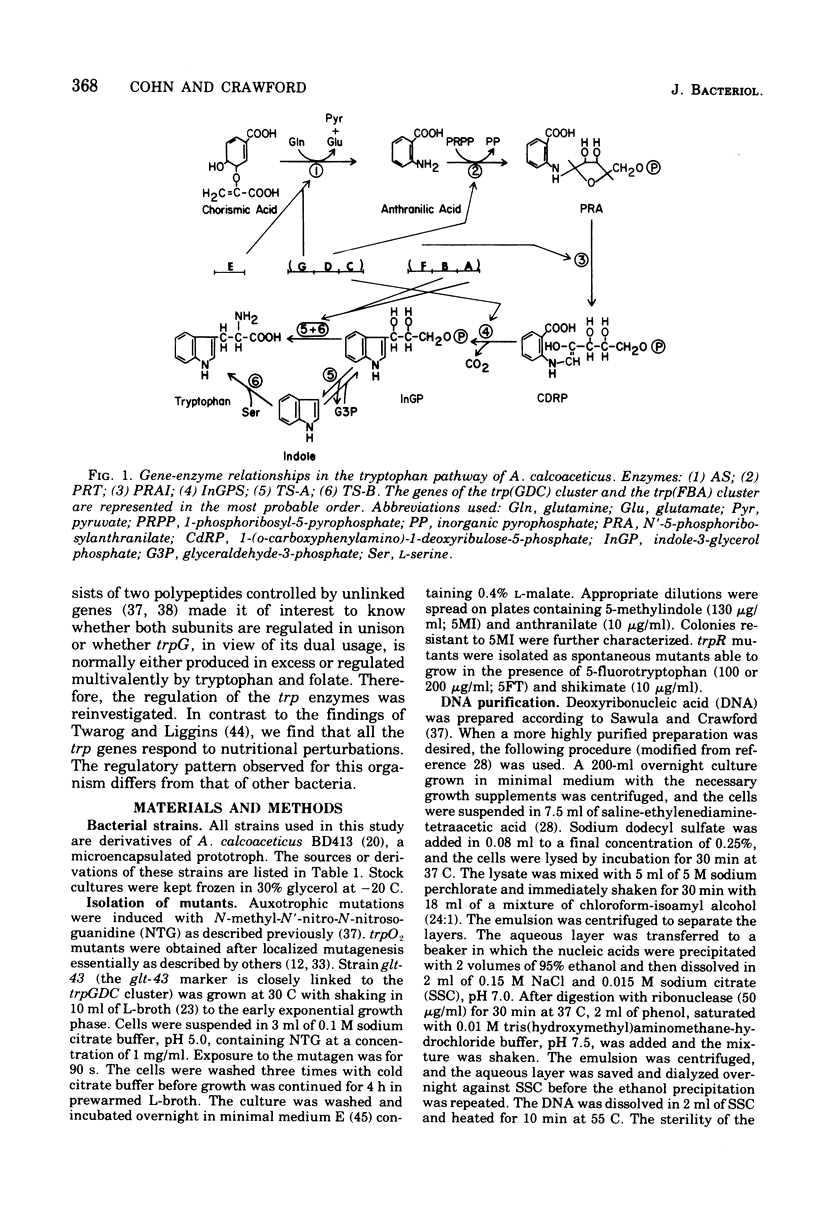
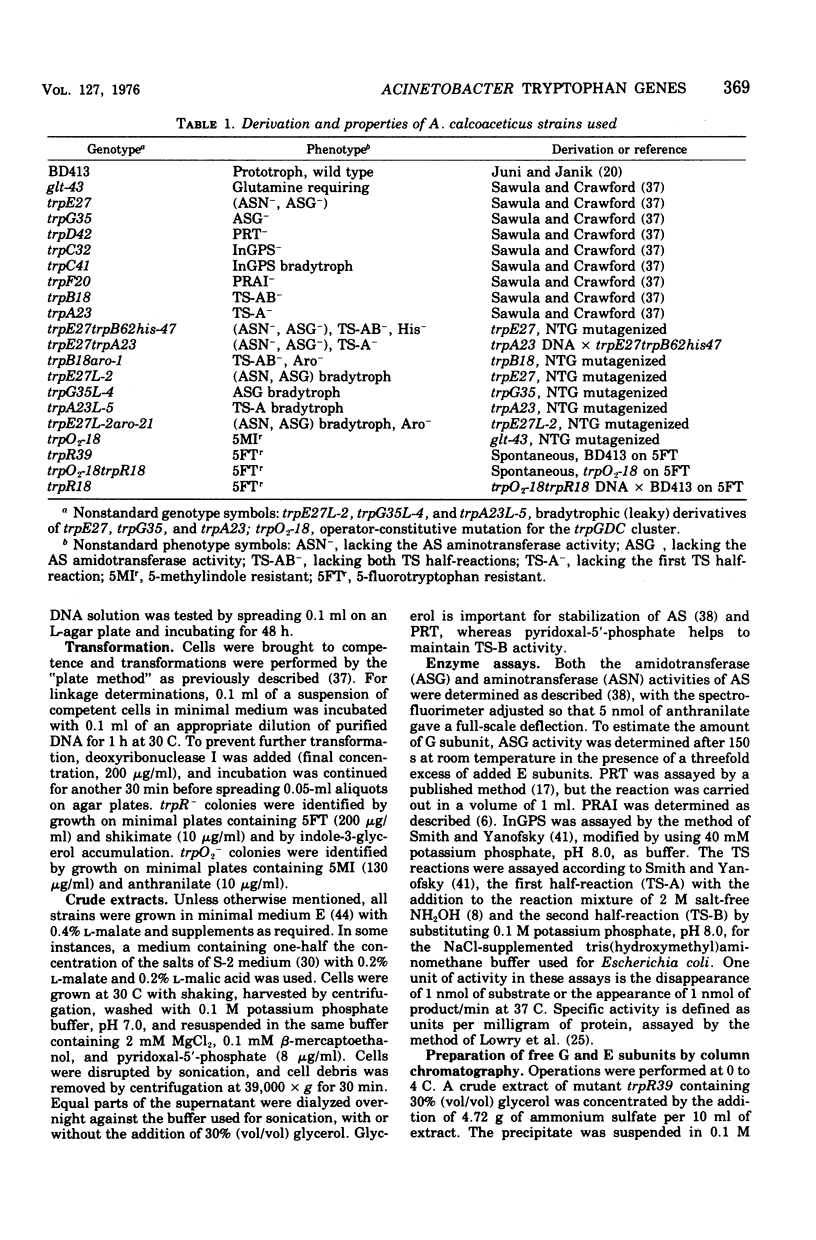
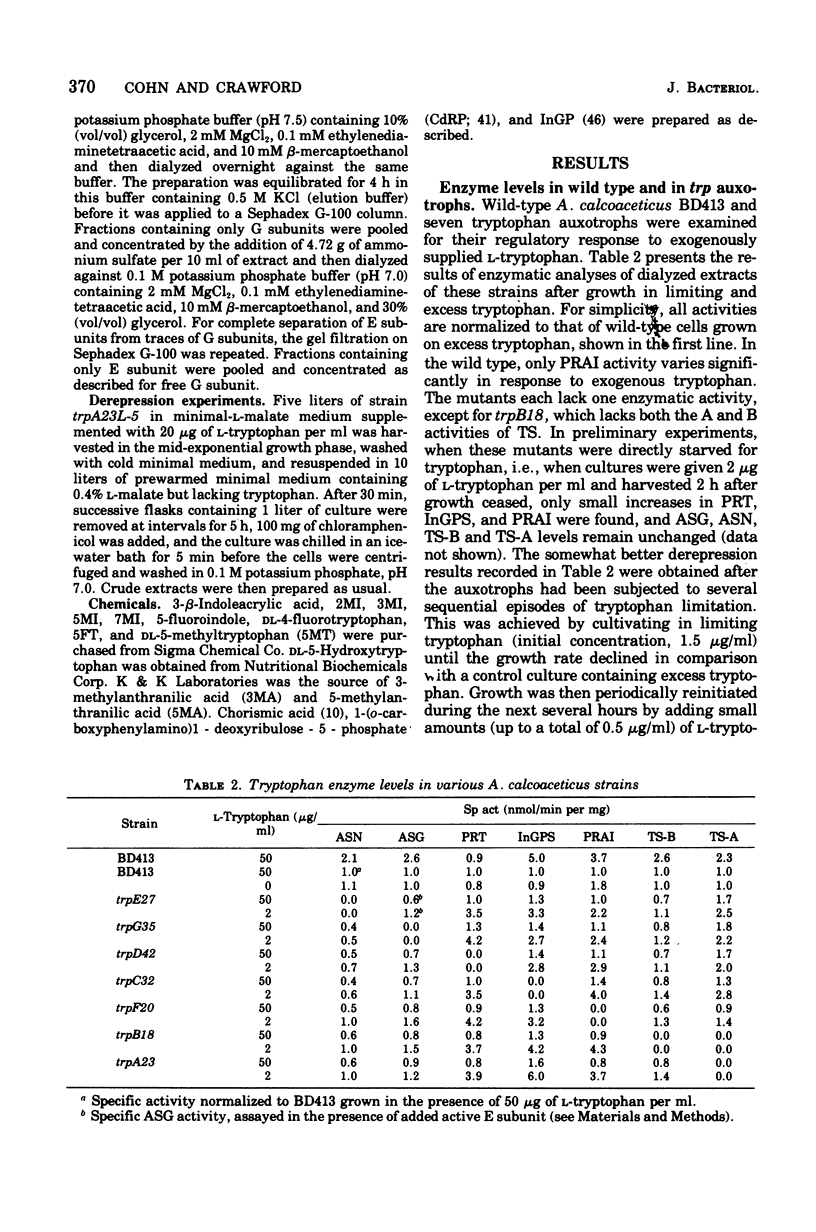
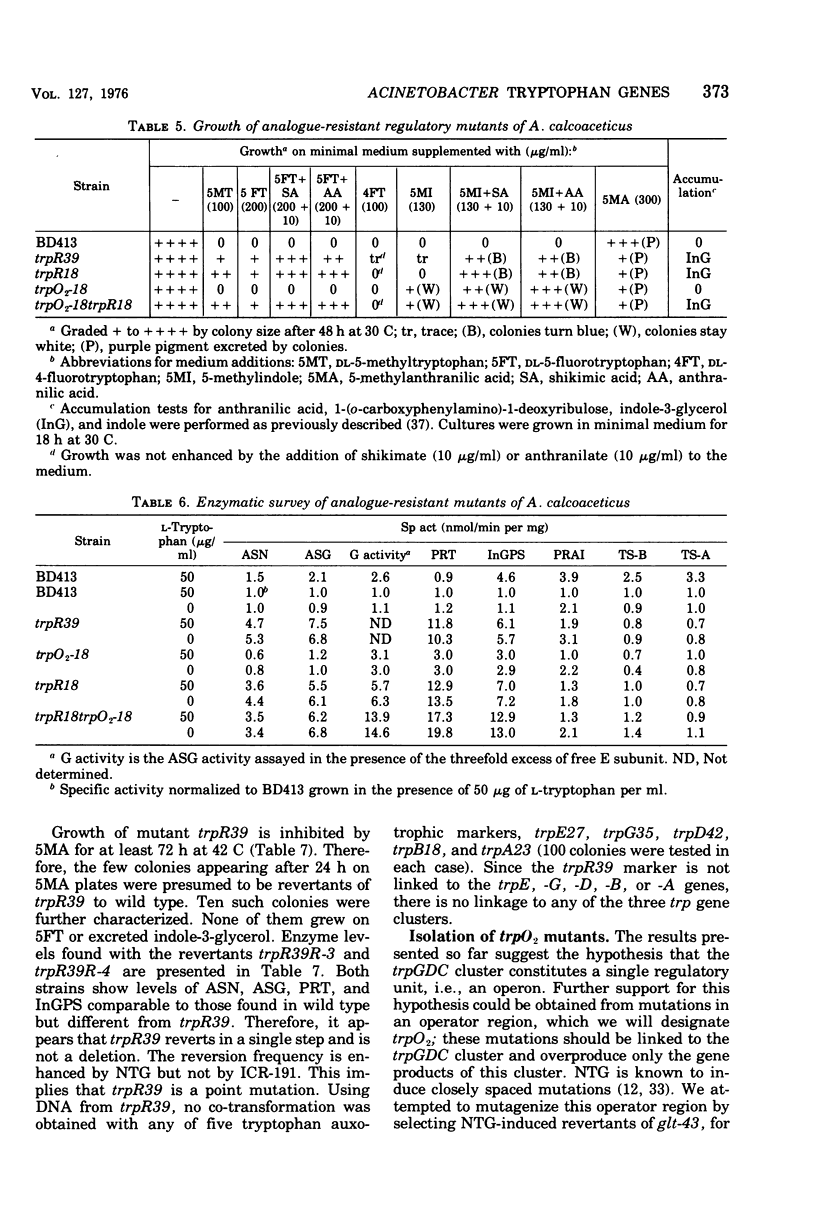
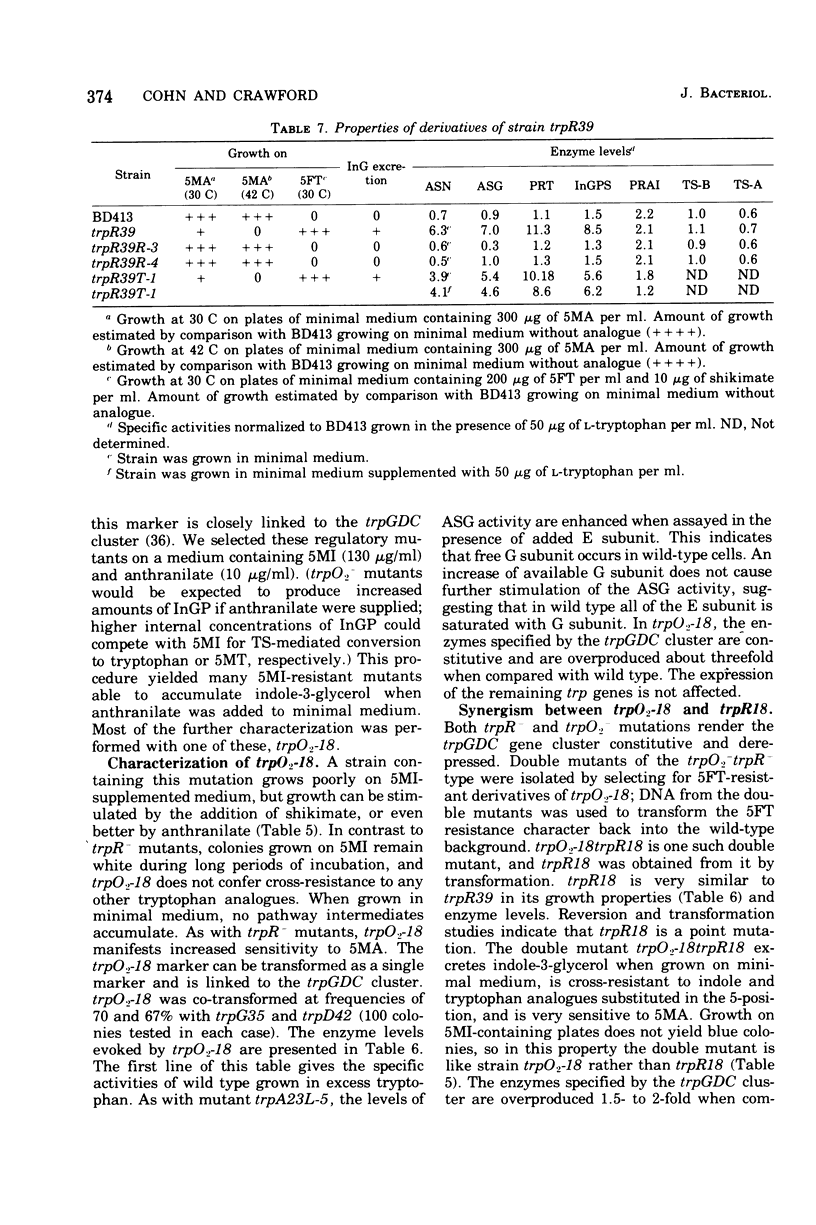
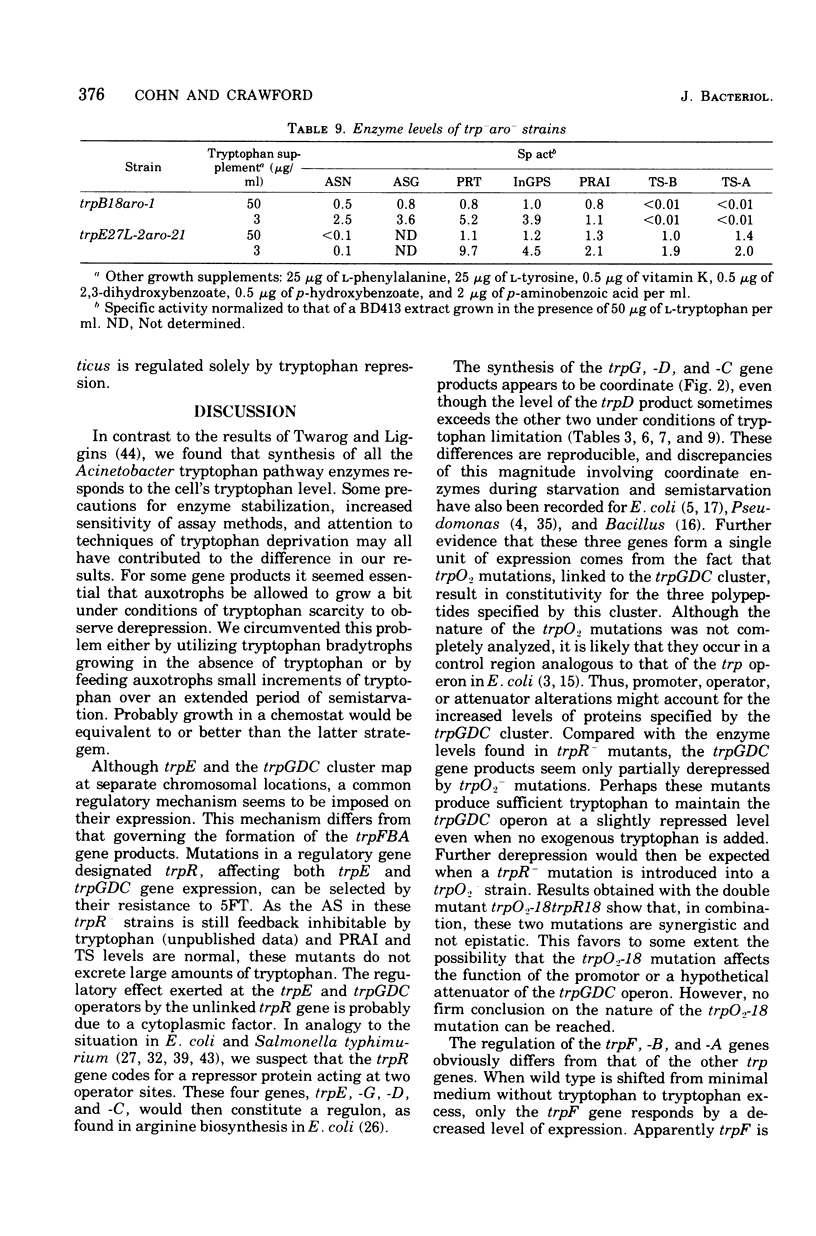
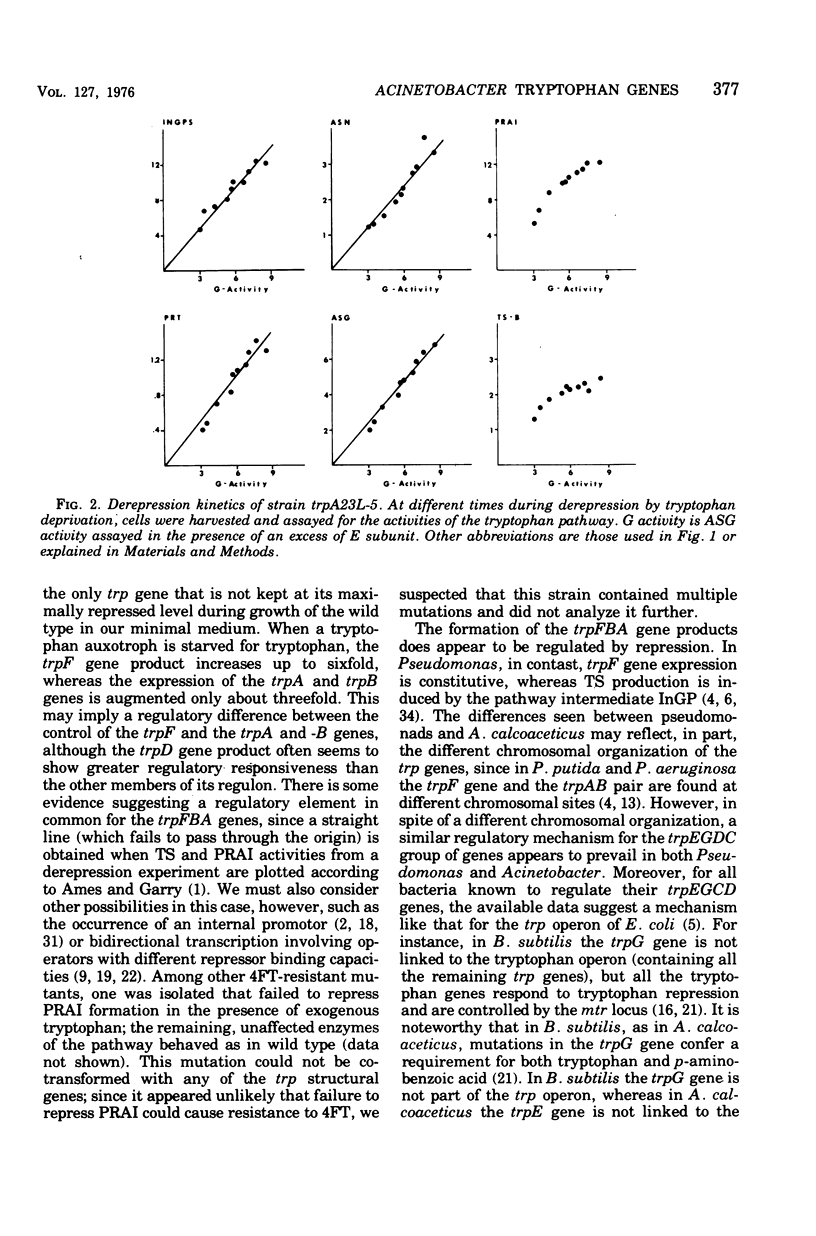
In Acinetobacter calcoaceticus the seven genes coding for the enzymes responsible for tryptophan synthesis map at three chromosomal locations. Two three-gene clusters, one (trpGDC) specifying the small subunit of anthranilate synthase, phosphoribosyl transferase, and indoleglycerol phosphate synthase and the other (trpFBA) specifying phosphoribosyl anthranilate isomerase and both tryptophan synthase subunits, are not linked to each other or to the trpE gene specifying the large anthranilate synthase subunit. When regulation of trp gene expression is studied in the wild type, only the level of the trpF gene product decreases upon addition of tryptophan to the medium. Tryptophan starvation of tryptophan auxotrophs, however, results in increased levels of all the tryptophan enzymes; this and additional evidence suggests that the expression of all the trp genes is subject to repression. The trpGDC genes are coordinately controlled, and the trpE gene is regulated in parallel with them. The trpFBA genes are controlled neither coordinately nor in parallel with the other trp genes, but respond proportionally when compared with each other. So far, two types of constitutive mutants have been found. The first class of mutants apparently occurs in the structural gene for a repressor protein; this repressor locus is unlinked to any of the biosynthetic trp genes and affects only the expression of trpE and the trpGDC cluster. The second class contains mutants closely linked to the trpGDC region; they overproduce only the gene products of this cluster.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. The functional organization of the tryptophan gene cluster in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1966 Jul;56(1):111–118. doi: 10.1073/pnas.56.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. The regulation of tryptophan biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1973 Mar 1;121(2):117–132. doi: 10.1007/BF00277526. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. N-(5'-phosphoribosyl)anthranilate isomerase-indol-3-ylglycerol phosphate synthetase of tryptophan biosynthesis. Relationship between the two activities of the enzyme from Escherichia coli. Biochem J. 1970 Dec;120(4):699–707. doi: 10.1042/bj1200699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D., Cunin R., Glansdorff N. Control regions within the argECBH gene cluster of Escherichia coli K12. Mol Gen Genet. 1972;117(4):349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Gunsalus C., Gunsalus C. F., Chakrabarty A. M., Sikes S., Crawford I. P. Fine structure mapping of the tryptophan genes in Pseudomonas putida. Genetics. 1968 Nov;60(3):419–435. doi: 10.1093/genetics/60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen S. D. Moraxella, Acinetobacter, and the Mimeae. Bacteriol Rev. 1973 Dec;37(4):522–561. doi: 10.1128/br.37.4.522-561.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. Operator mutants of the tryptophan operon in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):159–179. doi: 10.1016/0022-2836(69)90340-4. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Roth C. W., Crawford I. P., Nester E. W. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):38–45. doi: 10.1128/jb.105.1.38-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Crawford I. P. Regulation of the enzymes of the tryptophan pathway in Escherichia coli. Genetics. 1965 Dec;52(6):1303–1316. doi: 10.1093/genetics/52.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;117(4):337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. F., Holmes W. M., Jensen R. A. Metabolic interlock. The dual function of a folate pathway gene as an extra-operonic gene of tryptophan biosynthesis. J Biol Chem. 1972 Mar 10;247(5):1587–1596. [PubMed] [Google Scholar]
- Ketner G., Campbel A. Operator and promoter mutations affecting divergent transcription in the bio gene cluster of Escherichia coli. J Mol Biol. 1975 Jul 25;96(1):13–27. doi: 10.1016/0022-2836(75)90179-5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li S. S., Hanlon J., Yanofsky C. Amino-terminal sequences of indoleglycerol phosphate synthetase of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1975 Aug;123(2):761–764. doi: 10.1128/jb.123.2.761-764.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- Maurer R., Crawford I. P. New regulatory mutation affecting some of the tryptophan genes in Pseudomonas putida. J Bacteriol. 1971 May;106(2):331–338. doi: 10.1128/jb.106.2.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Amber mutants of the trpR regulatory gene. J Mol Biol. 1969 Aug 28;44(1):185–193. doi: 10.1016/0022-2836(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. The internal low-efficiency promoter of the tryptophan operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):447–451. doi: 10.1016/0022-2836(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Berlyn M. K. A simple procedure for localized mutagenesis using nitrosoguanidine. Mol Gen Genet. 1974;134(1):77–83. doi: 10.1007/BF00332814. [DOI] [PubMed] [Google Scholar]
- Proctor A. R., Crawford I. P. Autogenous regulation of the inducible tryptophan synthase of Pseudomonas putida. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1249–1253. doi: 10.1073/pnas.72.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queener S. W., Queener S. F., Meeks J. R., Gunsalus I. C. Anthranilate synthase from Pseudomonas putida. Purification and properties of a two-component enzyme. J Biol Chem. 1973 Jan 10;248(1):151–161. [PubMed] [Google Scholar]
- Reiners J. J., Jr, Zalkin H. Immunological study of anthranilate synthetase. J Bacteriol. 1975 Aug;123(2):620–630. doi: 10.1128/jb.123.2.620-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Anthranilate synthetase of Acinetobacter calcoaceticus. Separation and partial characterization of subunits. J Biol Chem. 1973 May 25;248(10):3573–3581. [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Mapping of the tryptophan genes of Acinetobacter calcoaceticus by transformation. J Bacteriol. 1972 Nov;112(2):797–805. doi: 10.1128/jb.112.2.797-805.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shimizu N., Hayashi M. In vitro repression of transcription of the tryptophan operon by trp repressor. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1990–1994. doi: 10.1073/pnas.70.7.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith O. H. Structure of the trpC cistron specifying indoleglycerol phosphate synthetase, and its localization in the tryptophan operon of Escherichia coli. Genetics. 1967 Sep;57(1):95–105. doi: 10.1093/genetics/57.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. L., Rose J. K., Yanofsky C., Yang H. L., Zubay G. Tryptophanyl-tRNA and tryptophanyl-tRNA synthetase are not required for in vitro repression of the tryptophan operon. Nat New Biol. 1973 Oct 3;245(144):131–133. doi: 10.1038/newbio245131a0. [DOI] [PubMed] [Google Scholar]
- Twarog R., Liggins G. L. Enzymes of the tryptophan pathway in Acinetobacter calco-aceticus. J Bacteriol. 1970 Oct;104(1):254–263. doi: 10.1128/jb.104.1.254-263.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wegman J., Crawford I. P. Tryptophan synthetic pathway and its regulation in Chromobacterium violaceum. J Bacteriol. 1968 Jun;95(6):2325–2335. doi: 10.1128/jb.95.6.2325-2335.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



