Abstract
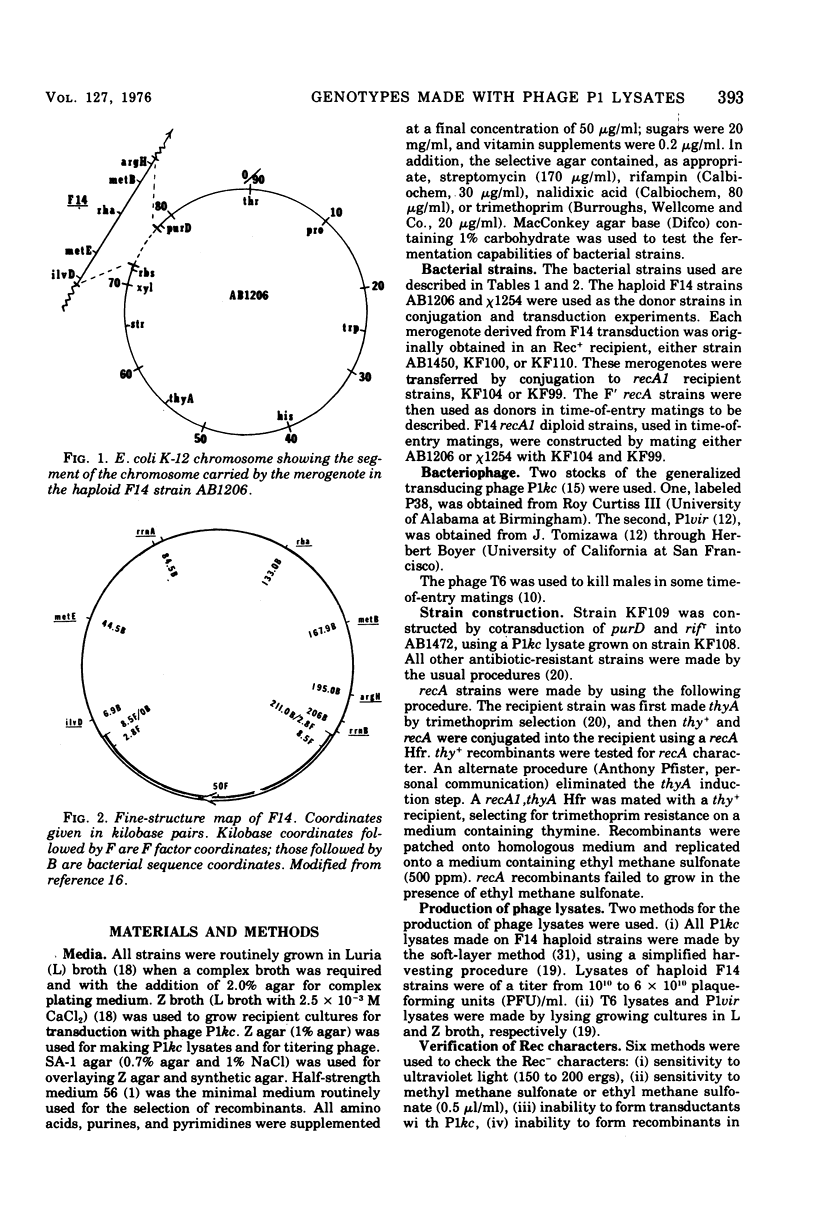
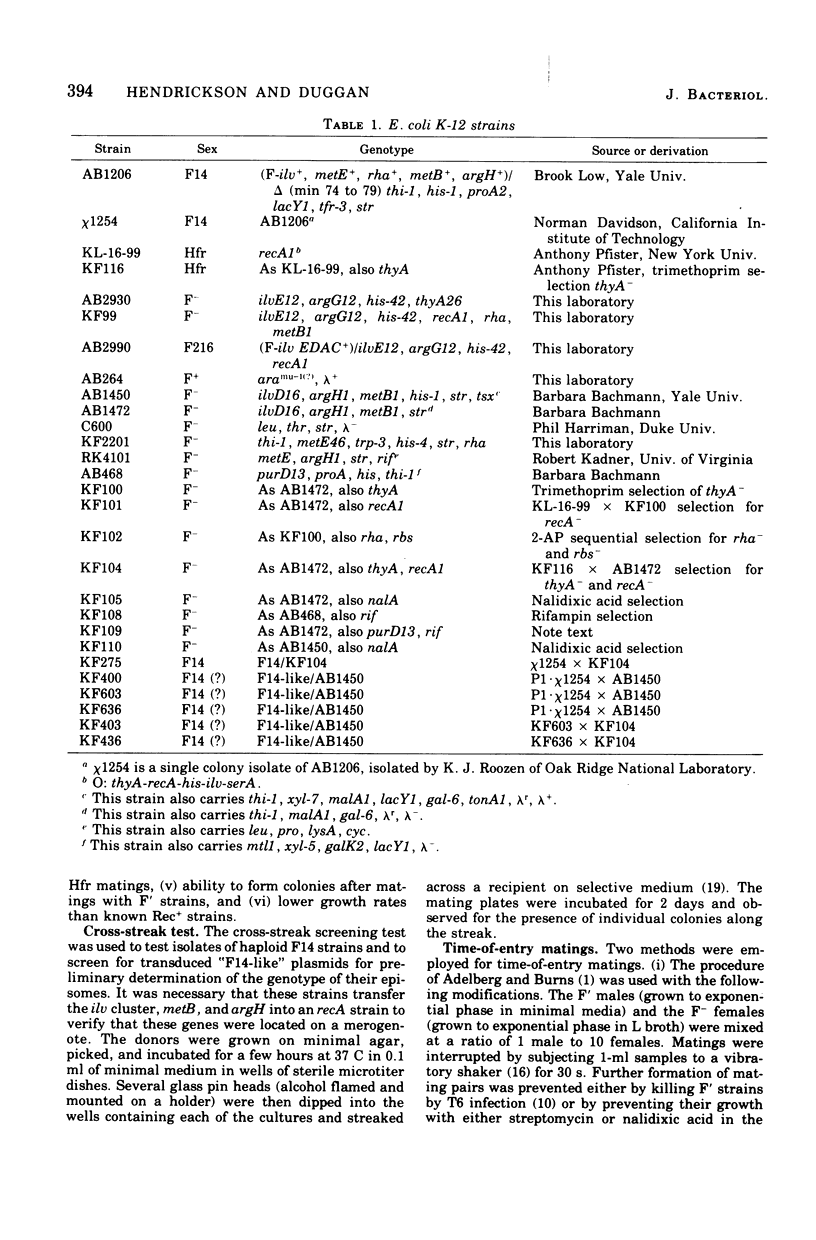
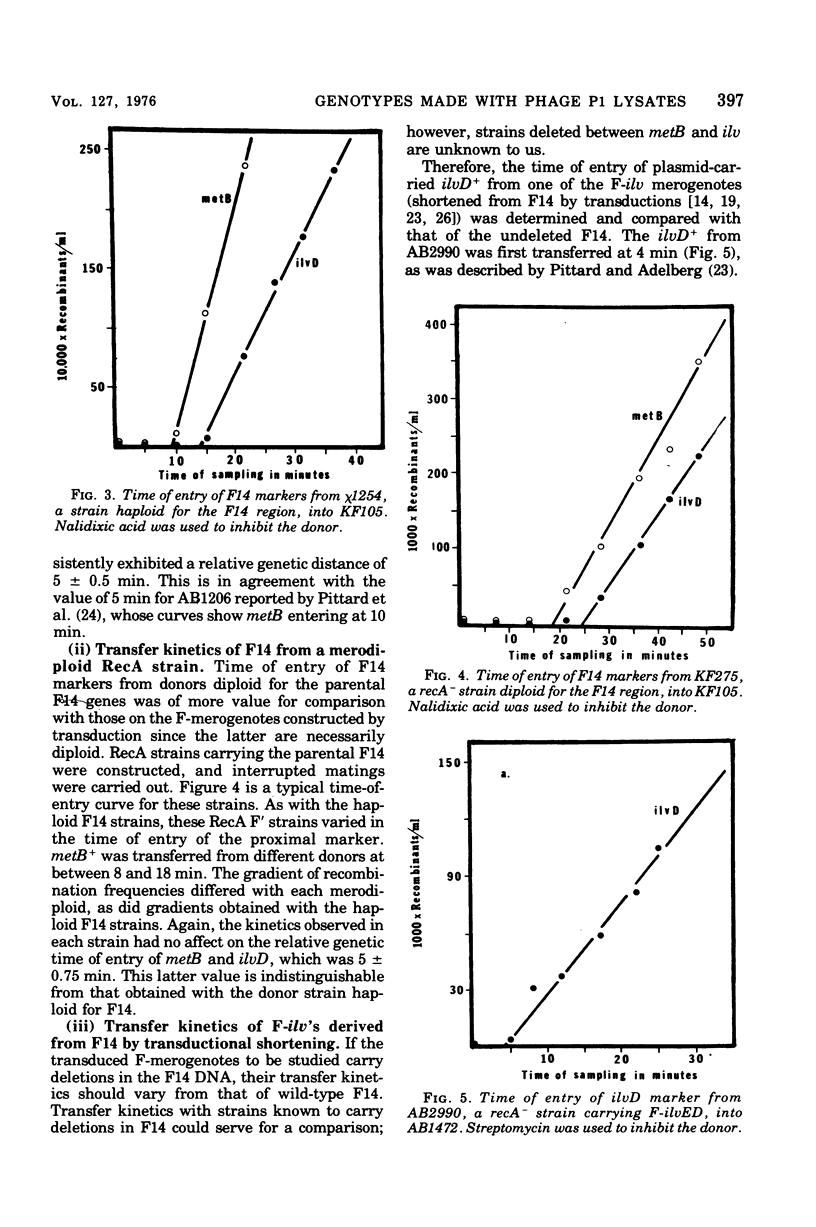
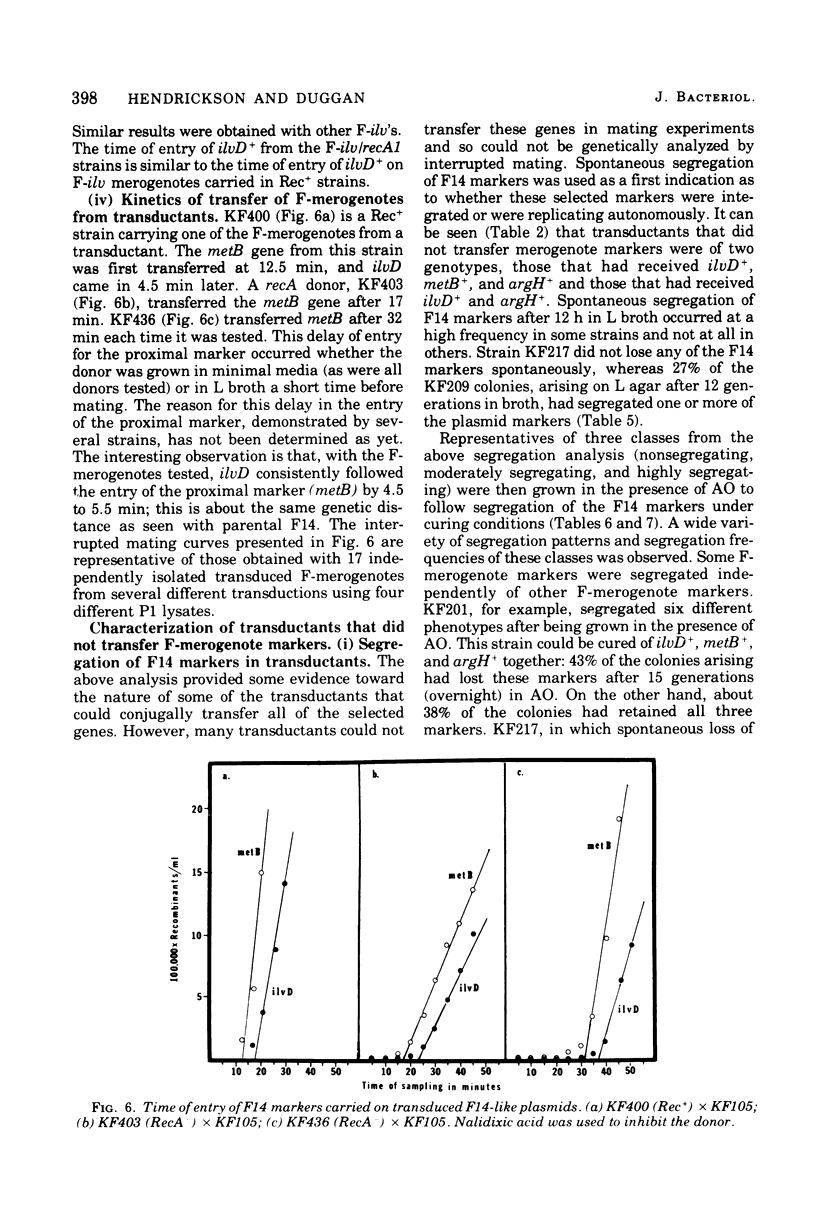
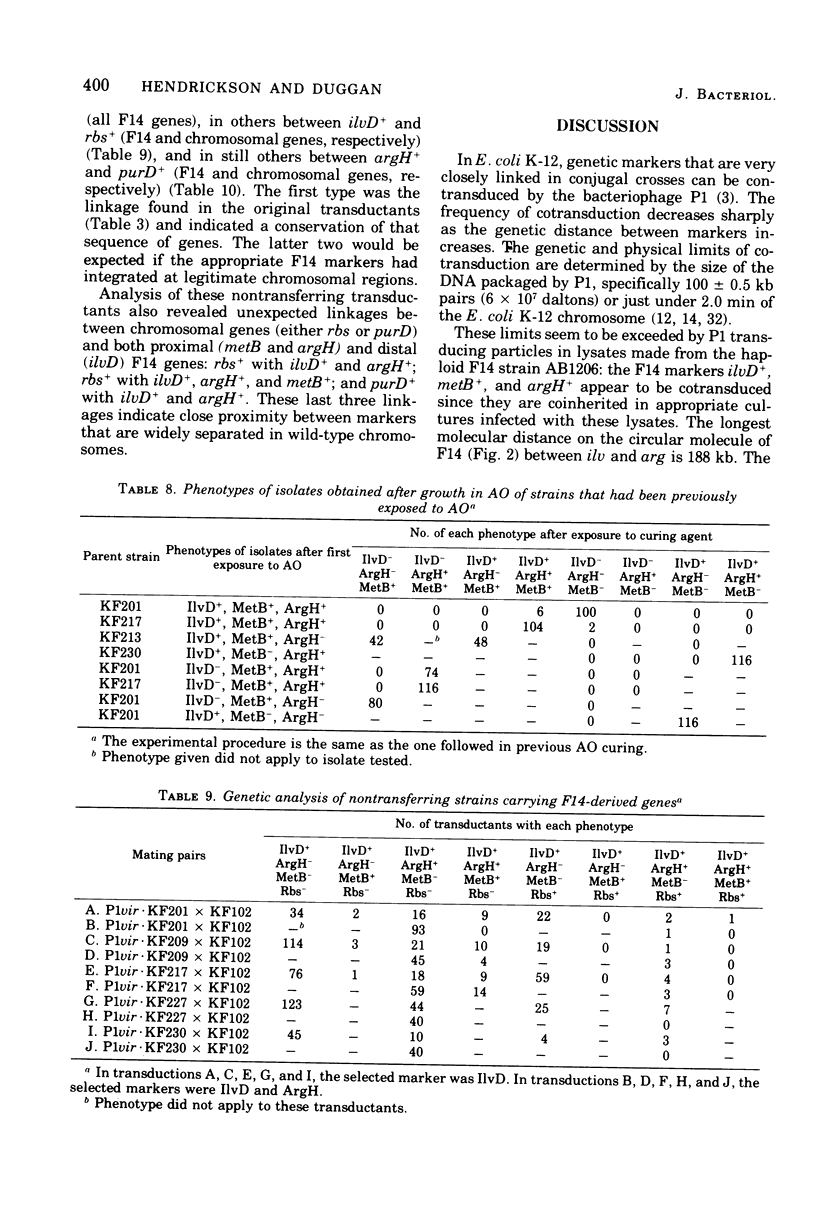
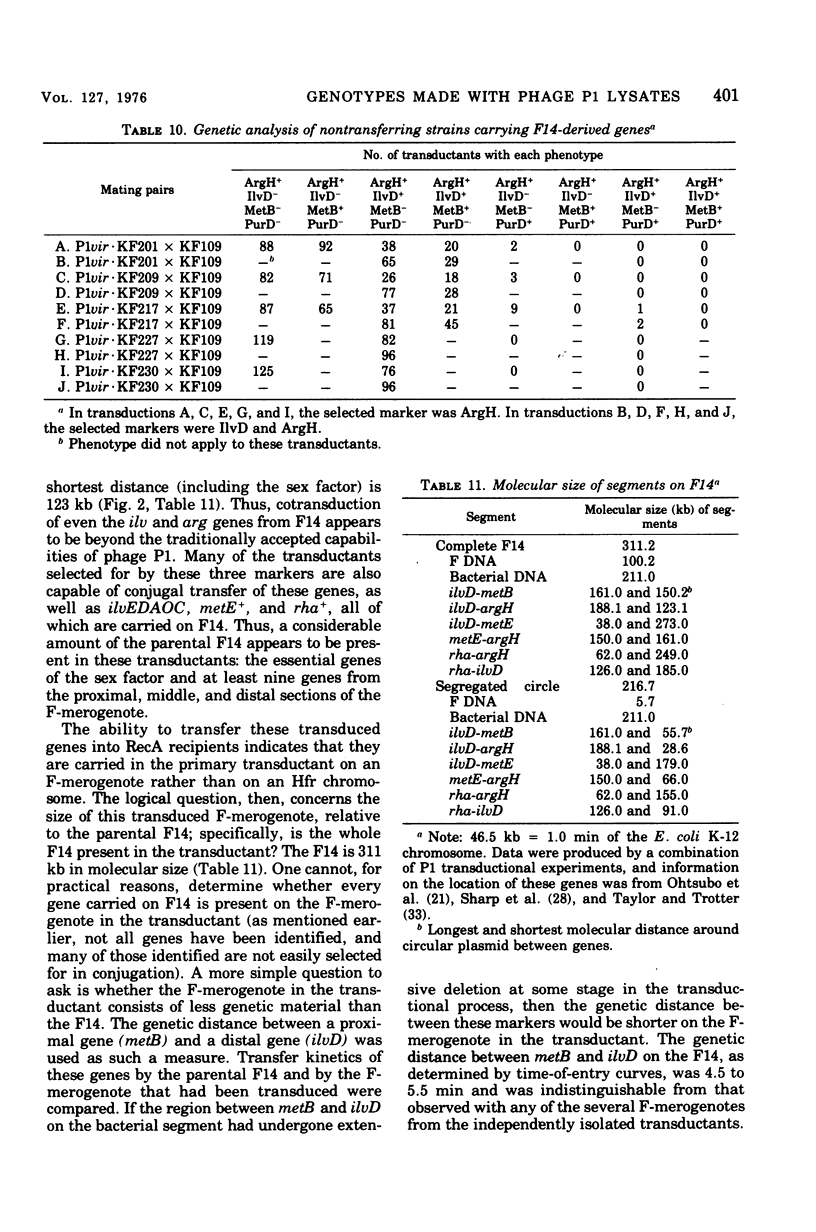
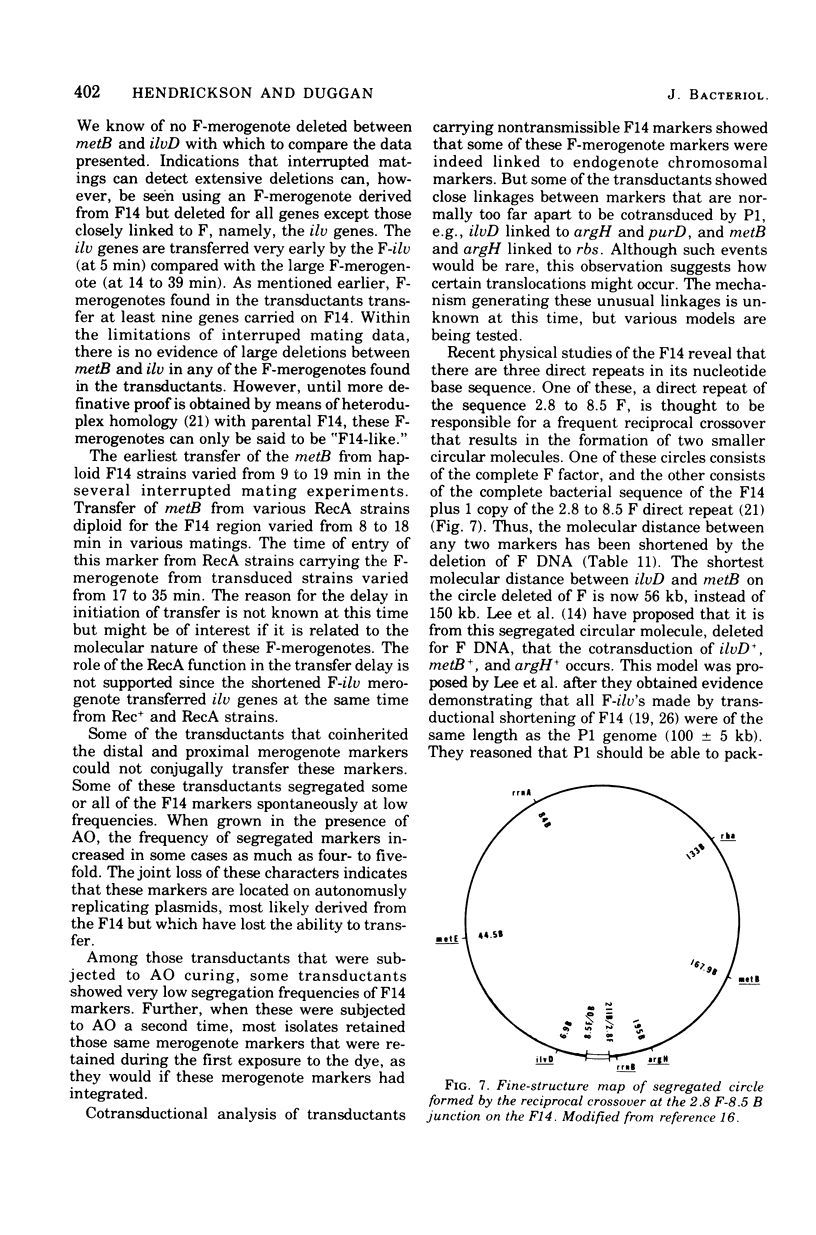
Among P1 transductants in Escherichia coli K-12 that were selected for the proximal and distal markers from the large F14 merogenote, a variety of unusual genotypes were found. As earlier workers had found, one class of these could transfer the proximal genes (argH, metB) and distal genes (ilvEDAC) of the F14 during conjugation. These F14 genes could be transferred into RecA recipients, indicating that they were carried on an F-merogenote rather than on an Hfr chromosome. The transduced F-merogenotes could transfer other F14 genes (metE, rha) as well. Transfer kinetic analysis showed that all of the latter transduced F-merogenotes that were examined were indistinguishable from the parental F14 in the order of transfer and the genetic distance between proximal and distal markers. This suggests that the whole F14 had been received somehow by the primary transductional recipients, a remarkable possibility since the F14 is much larger than the largest deoxyribonucleic acid segment normally transduced by P1. The mechanism of this phenomenon is not yet known. Many of the transductants did not transfer any of the F14 markers tested. Some of these transductants segregated certain F14 genes, indicating they were carried on self-replicating genetic elements, but others were not cured of F14 markers, even by acridine orange. Cotransductional analysis of this group was consistent with the hypothesis that the F14 markers in some of these strains had integrated into the chromosome in the expected manner, since in these latter the F14 alleles were linked to the expected chromosomal genes. Other strains among the stable transductants had acquired new linkages in that genes previously separated by several minutes could now be cotransduced. These latter included the novel cotransductional linkages of rbs-ilv-argH, rbs-ilv-argH-metB, and ilvD-argH-purD. Such strains might have been formed as a result of insertion into the chromosome of small circles derived from F14.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S. J., Tittawella I. P., Hayward R. S., Scaife J. G. Amber mutations of Escherichia coli RNA polymerase. Nat New Biol. 1971 Aug 4;232(31):133–136. doi: 10.1038/newbio232133a0. [DOI] [PubMed] [Google Scholar]
- Barbour S. D. Effect of nalidixic acid on conjugational transfer and expression of episomal lac genes in Escherichia coli K12. J Mol Biol. 1967 Sep 14;28(2):373–376. doi: 10.1016/s0022-2836(67)80016-0. [DOI] [PubMed] [Google Scholar]
- Bouck N., Adelberg E. A. Mechanism of action of nalidixic acid on conjugating bacteria. J Bacteriol. 1970 Jun;102(3):688–701. doi: 10.1128/jb.102.3.688-701.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S., Couse N. L. Structural aberrations in T-even bacteriophage. 3. Induction of "lollipops" and their partial characterization. Virology. 1973 Jul;54(1):245–261. doi: 10.1016/0042-6822(73)90134-7. [DOI] [PubMed] [Google Scholar]
- Doermann A. H., Eiserling F. A., Boehner L. Genetic control of capsid length in bacteriophage T4. I. Isolation and preliminary description of four new mutants. J Virol. 1973 Aug;12(2):374–385. doi: 10.1128/jvi.12.2.374-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES W. The kinetics of the mating process in Escherichia coli. J Gen Microbiol. 1957 Feb;16(1):97–119. doi: 10.1099/00221287-16-1-97. [DOI] [PubMed] [Google Scholar]
- Harriman P. D. A single-burst analysis of the production of P1 infectious and transducing particles. Virology. 1972 May;48(2):595–600. doi: 10.1016/0042-6822(72)90071-2. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Otsubo E., Deonier R. C., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. V. ilv+ Deletion mutants of F14. J Mol Biol. 1974 Nov 15;89(4):585–597. doi: 10.1016/0022-2836(74)90037-0. [DOI] [PubMed] [Google Scholar]
- Marsh N. J., Duggan D. E. Ordering of mutant sites in the isoleucine-valine genes of Escherichia coli by use of merogenotes derived from F 14 : a new procedure for fine-structure mapping. J Bacteriol. 1972 Feb;109(2):730–740. doi: 10.1128/jb.109.2.730-740.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E., Deonier R. C., Lee H. J., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. IV. The F sequences in F14. J Mol Biol. 1974 Nov 15;89(4):565–584. doi: 10.1016/0022-2836(74)90036-9. [DOI] [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. II. INTERACTION BETWEEN F-MEROGENOTE AND CHROMOSOME DURING TRANSFER. J Bacteriol. 1963 Jun;85:1402–1408. doi: 10.1128/jb.85.6.1402-1408.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., LOUTIT J. S., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. I. DELAY IN INITIATION OF CHROMOSOME TRANSFER. J Bacteriol. 1963 Jun;85:1394–1401. doi: 10.1128/jb.85.6.1394-1401.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae M. E., Stodolsky M. Chromosome breakage, fusion and reconstruction during P1dl transduction. Virology. 1974 Mar;58(1):32–54. doi: 10.1016/0042-6822(74)90139-1. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Specialized transduction of pro genes by coliphage P1: structure of a partly diploid P1-pro prophage. Virology. 1975 Sep;67(1):42–55. doi: 10.1016/0042-6822(75)90401-8. [DOI] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Stodolsky M. Chromosome fusion. Genetics. 1973 Apr;73:73–73:66. [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Jr, Anderson T. F. Morphological variants of coliphage P1. J Virol. 1970 Jun;5(6):765–782. doi: 10.1128/jvi.5.6.765-782.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



