Abstract
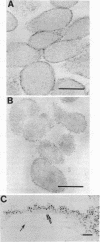
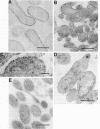
Anionic sites on mycoplasma membranes were visualized in the electron microscope by a polycationized ferritin derivative. The technique of thin sectioning was used. Staining prior to fixation led to clustering of ferritin granules on the mycoplasma cell surface. On glutaraldehyde-fixed Mycoplasma mycoides subsp. capri, M. gallisepticum, M. pneumoniae, and Acholeplasma laidlawii, the anionic sites were uniformly distributed over the entire membrane surface. M. hominis did not bind the polycationic ferritin label. Chemical and enzymatic treatments of the mycoplasmas indicated that the anionic sites may be lipid phosphate groups. Isolated M. mycoides subsp. capri membranes were labeled exclusively on only one membrane surface, presumably the outer one. Liposomes prepared from diphosphatidylglycerol and phosphatidylcholine were also labeled by the polycationic ferritin.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Fraser D., Fleischmann C. Interaction of mycoplasma with viruses. I. Primary adsorption of virus is ionic in mechanism. J Virol. 1974 May;13(5):1067–1074. doi: 10.1128/jvi.13.5.1067-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic G. J., Berwick L., Sorrentino M. Positive and negative colloidal iron as cell surface electron stains. Lab Invest. 1968 Jan;18(1):63–71. [PubMed] [Google Scholar]
- Grinnell F., Tobleman M. Q., Hackenbrock C. R. The distribution and mobility of anionic sites on the surfaces of baby hamster kidney cells. J Cell Biol. 1975 Sep;66(3):470–479. doi: 10.1083/jcb.66.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R., Miller K. J. The distribution of anionic sites on the surfaces of mitochondrial membranes. Visual probing with polycationic ferritin. J Cell Biol. 1975 Jun;65(3):615–630. doi: 10.1083/jcb.65.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkate F., van Deenen L. L. Isolation and chemical characterization of phosphatidyl glycerol from spinach leaves. Biochim Biophys Acta. 1965 Jul 7;106(1):78–92. doi: 10.1016/0005-2760(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Huang R. T. Adsorption of influenza virus to charged groups on natural and artificial surfaces. Med Microbiol Immunol. 1974;159(2):129–135. doi: 10.1007/BF02123725. [DOI] [PubMed] [Google Scholar]
- JAMES A. M. The electrochemistry of the bacterial surface. Prog Biophys Biophys Chem. 1957;8:95–142. [PubMed] [Google Scholar]
- Kahane I., Ne'eman Z., Razin S. Divalent cations in native and reaggregated mycoplasma membranes. J Bacteriol. 1973 Feb;113(2):666–671. doi: 10.1128/jb.113.2.666-671.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M. Osmolar concentration and fixation of mycoplasmas. J Bacteriol. 1972 Jun;110(3):1154–1162. doi: 10.1128/jb.110.3.1154-1162.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Alteration of the conformation of proteins in red blood cell membranes and in solution by fixatives used in electron microscopy. J Cell Biol. 1968 Apr;37(1):117–121. doi: 10.1083/jcb.37.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew E. Complementarity between cell surfaces. J Theor Biol. 1974 Oct;47(2):483–484. doi: 10.1016/0022-5193(74)90210-0. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K. The bacterial surface. II. Effect of uranyl chloride on the electrophoretic mobility of bacteria. Biochim Biophys Acta. 1950 Sep;6(1):66–78. doi: 10.1016/0006-3002(50)90078-3. [DOI] [PubMed] [Google Scholar]
- Mehrishi J. N. Molecular aspects of the mammalian cell surface. Prog Biophys Mol Biol. 1972;25:1–70. doi: 10.1016/0079-6107(72)90013-2. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Anionic sites of human erythrocyte membranes. I. Effects of trypsin, phospholipase C, and pH on the topography of bound positively charged colloidal particles. J Cell Biol. 1973 May;57(2):373–387. doi: 10.1083/jcb.57.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond D. V., Fisher D. J. The electrophoretic mobility of micro-organisms. Adv Microb Physiol. 1973;9:1–29. doi: 10.1016/s0065-2911(08)60375-6. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hasin M., Razin S. Binding of proteins to mycoplasma membranes. Biochim Biophys Acta. 1973 Apr 16;298(4):876–886. doi: 10.1016/0005-2736(73)90392-1. [DOI] [PubMed] [Google Scholar]
- Rottem S., Hasin M., Razin S. Differences in susceptibility to phospholipase C of free and membrane-bound phospholipids of Mycoplasma hominis. Biochim Biophys Acta. 1973 Nov 16;323(4):520–531. doi: 10.1016/0005-2736(73)90160-0. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Isolation of mycoplasma membranes by digitonin. J Bacteriol. 1972 May;110(2):699–705. doi: 10.1128/jb.110.2.699-705.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Azzi A. Cation binding to submitochondrial particles. Biochim Biophys Acta. 1968 Apr 29;150(3):473–481. doi: 10.1016/0005-2736(68)90147-8. [DOI] [PubMed] [Google Scholar]
- Schiefer H. G., Gerhardt U., Brunner H. Immunological studies on the localization of phosphatidylglycerol in the membranes of Mycoplasma hominis. Hoppe Seylers Z Physiol Chem. 1975 May;356(5):559–565. doi: 10.1515/bchm2.1975.356.1.559. [DOI] [PubMed] [Google Scholar]
- Schiefer H. G., Gerhardt U., Brunner H., Krüpe M. Studies with lectins on the surface carbohydrate structures of mycoplasma membranes. J Bacteriol. 1974 Oct;120(1):81–88. doi: 10.1128/jb.120.1.81-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer H. G., Krauss H., Brunner H., Gerhardt U. Ultrastructural visualization of surface carbohydrate structures on mycoplasma membranes by concanavalin A. J Bacteriol. 1975 Dec;124(3):1598–1600. doi: 10.1128/jb.124.3.1598-1600.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer H. G., Schummer U., Hegner D., Gerhardt U., Schnepel G. H. Electron spin resonance studies on the lipid-protein interaction between cardiolipin and anti-cardiolipin antibodies. Hoppe Seylers Z Physiol Chem. 1975 Mar;356(3):293–299. doi: 10.1515/bchm2.1975.356.1.293. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. Mycoplasmas and cell cultures. Bacteriol Rev. 1971 Jun;35(2):206–227. doi: 10.1128/br.35.2.206-227.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subjeck J. R., Weiss L. The binding of cationized ferritin at the surfaces of ehrlich ascites tumor cells: the effect of pH and glutaraldehyde fixation. J Cell Physiol. 1975 Jun;85(3):529–536. doi: 10.1002/jcp.1040850304. [DOI] [PubMed] [Google Scholar]
- Weiss L., Mayhew E. Ribonuclease-susceptible charged groups at the surface of Ehrlich ascites tumour cells. Int J Cancer. 1969 Sep 15;4(5):626–635. doi: 10.1002/ijc.2910040507. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Colley C. M. Localization of red cell membrane constituents. Biochim Biophys Acta. 1973 Sep 10;300(2):159–182. doi: 10.1016/0304-4157(73)90003-8. [DOI] [PubMed] [Google Scholar]