Abstract
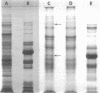
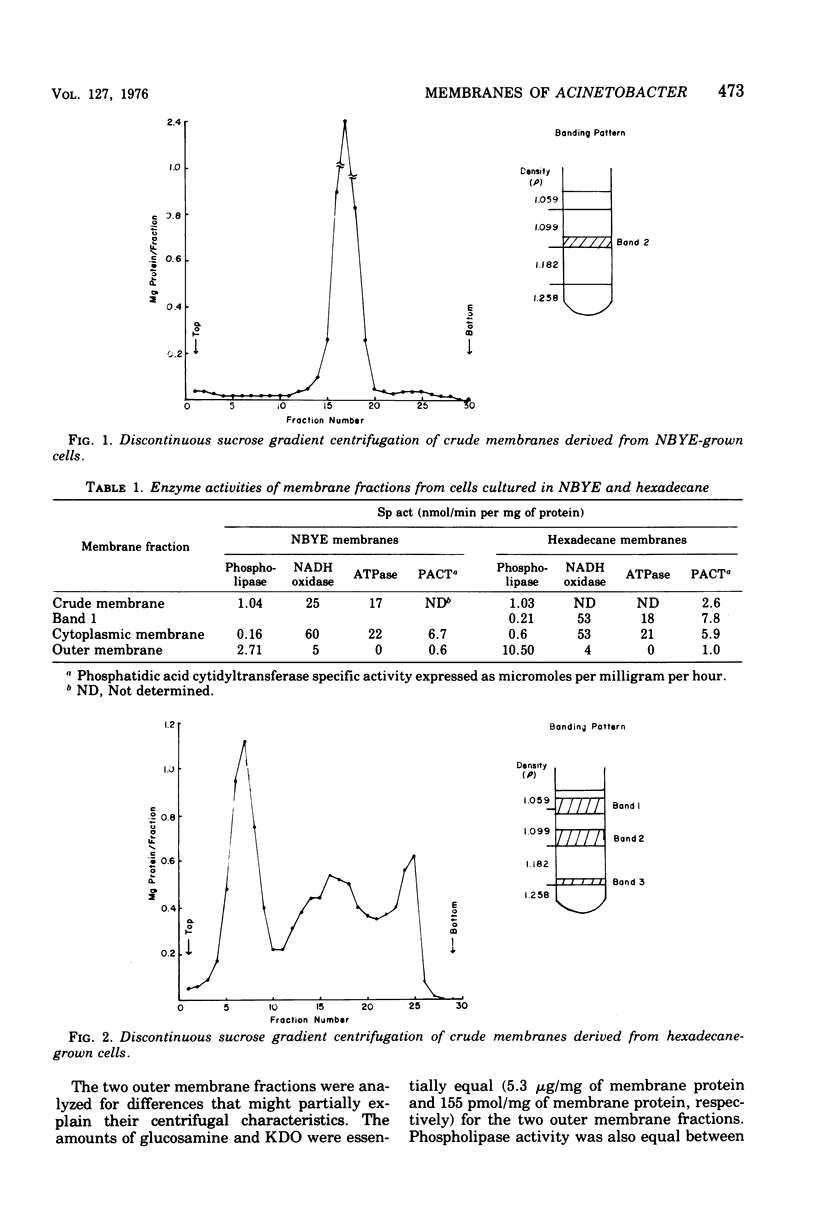
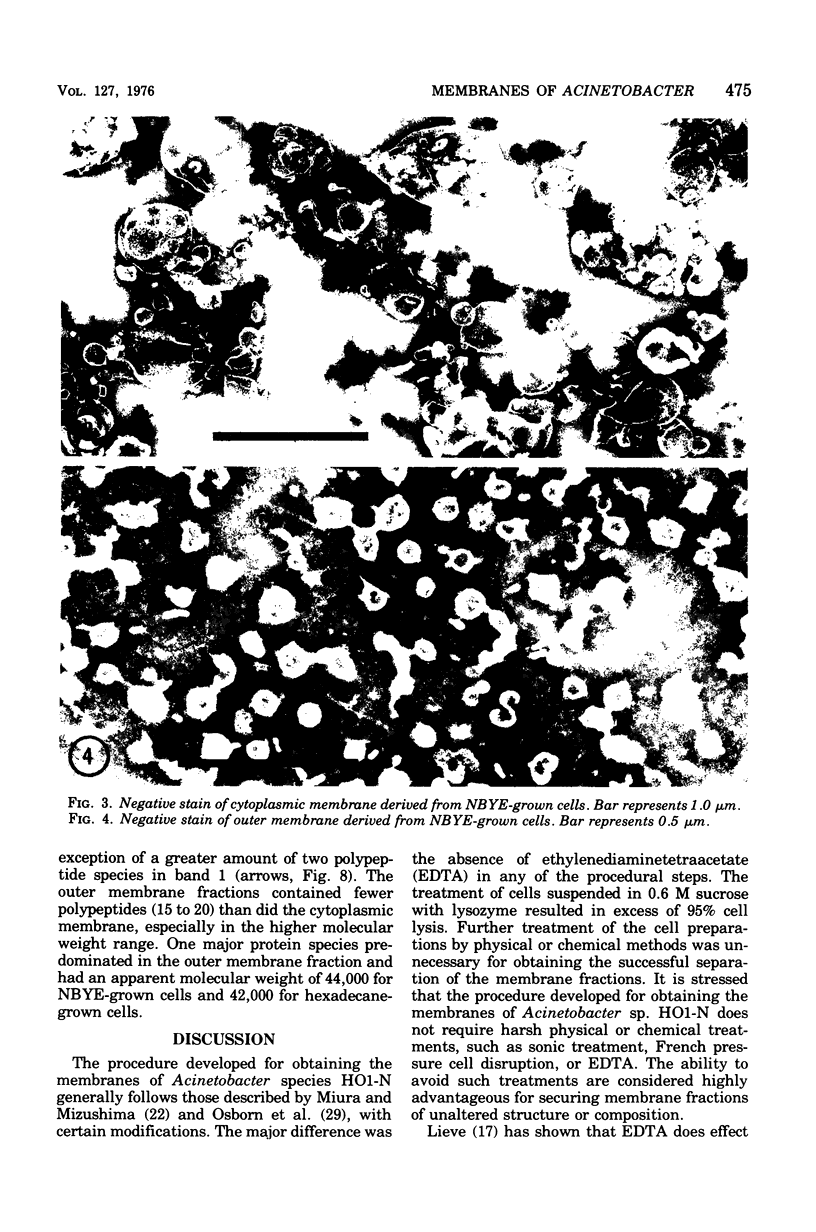
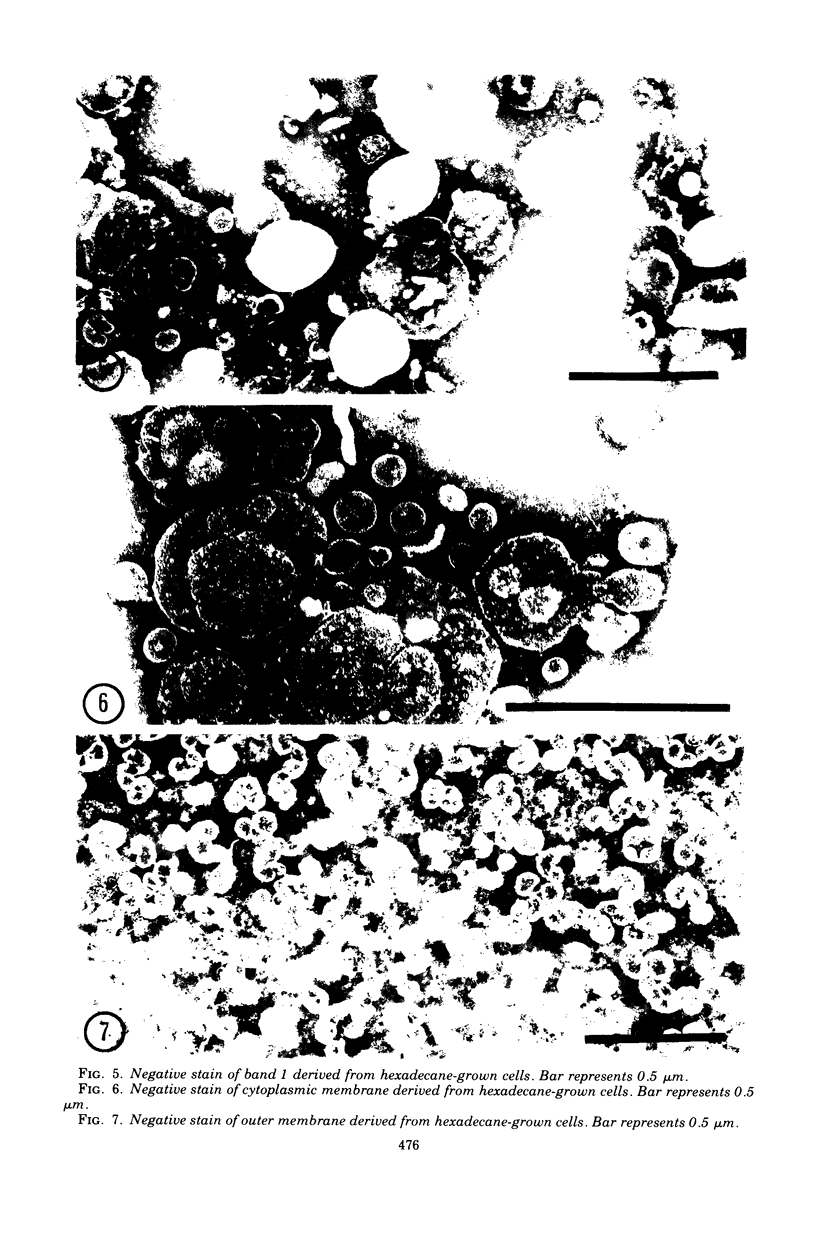
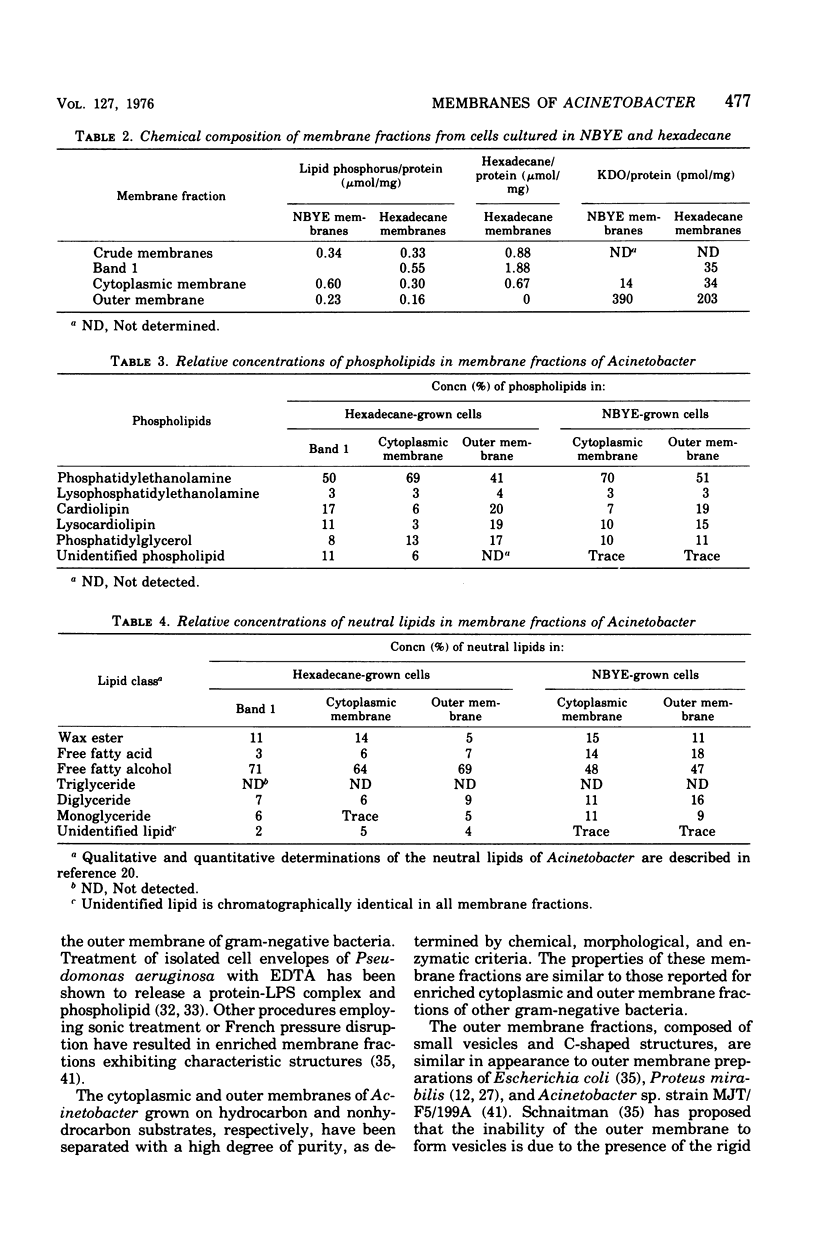
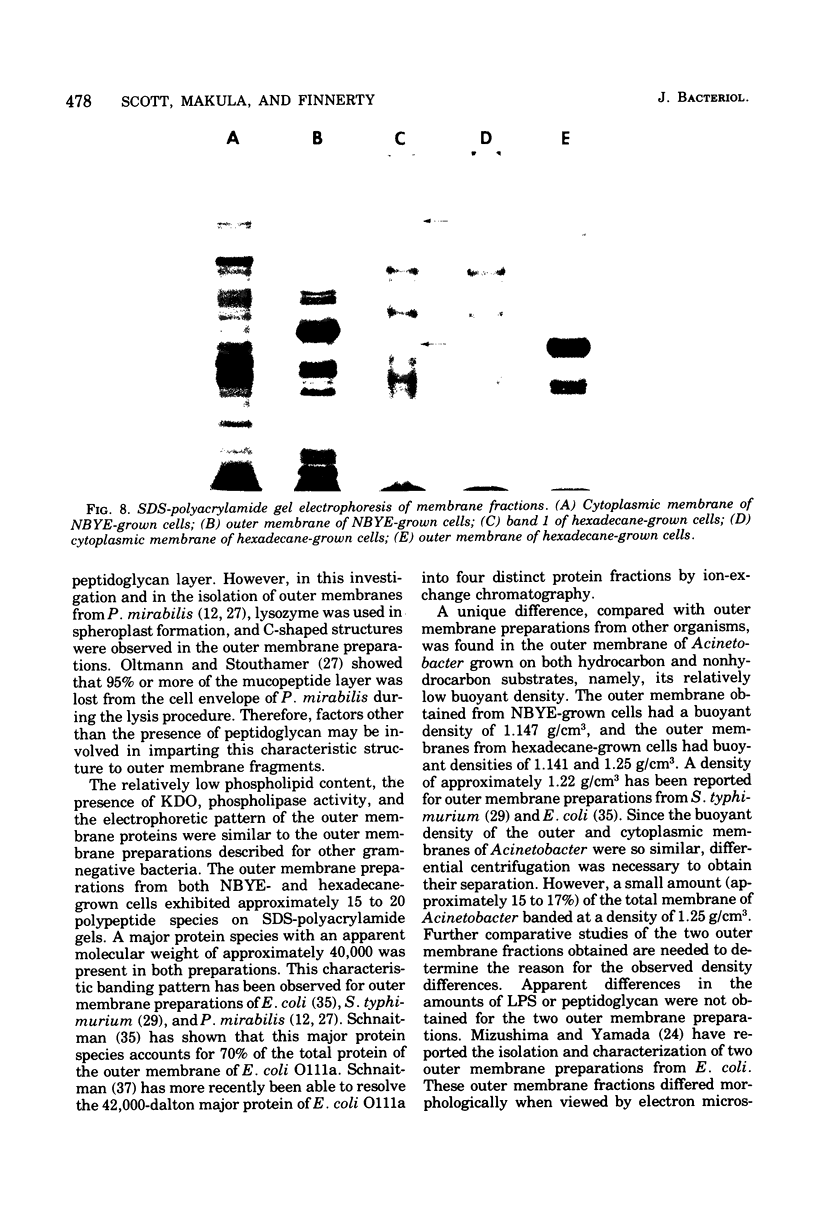
Membranes were isolated and purified from nutrient broth-yeast extract- and hexadecane-grown cells of Acinetobacter sp. strain HO1-N. Two membrane fractions were isolated from nutrient broth-yeast extract-grown cells, the cytoplasmic membrane and the outer membrane. In addition to these two membrane fractions, a unique membrane fraction was isolated from hexadecane-grown cells (band 1) and characterized as a lipid-rich, low-density membrane containing high concentrations of hexadecane. The outer membrane preparations of Acinetobacter, obtained from nutrient broth-yeast extract- and hexadecane-grown cells, exhibited a low ratio of lipid phosphorus to protein and contained phospholipase activity and 2-keto-3-deoxyoctulosonic acid. Phosphatidic acid cytidyltransferase, adenosine triphosphatase, and reduced nicotinamide adenine dinucleotide oxidase were recovered almost exclusively in the cytoplasmic membrane fractions. The cytoplasmic membrane fractions contained 20 to 25 polypeptide species on sodium dodecyl sulfate-polyacrylamide gels, and the outer membrane fractions contained 15 to 20 polypeptide species. A major polypeptide species with an apparent molecular weight of approximately 42,000 to 44,000 was found for all outer membrane fractions. The buoyant densities of the cytoplasmic membrane fractions and the outer membrane fractions were closely similar, necessitating their separation by differential centrifugation. Band 1 of hexadecane-grown cells had a ratio of lipid phosphorus to protein that was almost twice that of cytoplasmic membrane and a correspondingly low buoyant density (1.086 g/cm3). Enzyme activities associated with band 1 were identical to those associated with the cytoplasmic membrane. The electrophoretic banding pattern of band 1 was essentially identical to the banding pattern of the cytoplasmic membrane. The phospholipid and neutral lipid compositions of the isolated membrane fractions were determined as qualitatively similar, with significant quantitative differences. The ultrastructure characteristics of the respective membrane fractions were examined by the negative-stain technique.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Batzing B. L., Claus G. W. Fine structural changes of Acetobacter suboxydans during growth in a defined medium. J Bacteriol. 1973 Mar;113(3):1455–1461. doi: 10.1128/jb.113.3.1455-1461.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Claus G. W., Batzing B. L., Baker C. A., Goebel E. M. Intracytoplasmic membrane formation and increased oxidation of glycerol growth of Gluconobacter oxydans. J Bacteriol. 1975 Sep;123(3):1169–1183. doi: 10.1128/jb.123.3.1169-1183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. L., Whittenbury R. Fine structure of methane and other hydrocarbon-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):227–232. doi: 10.1099/00221287-61-2-227. [DOI] [PubMed] [Google Scholar]
- De Boer W. E., Hazeu W. Observations on the fine structure of a methane-oxidizing bacterium. Antonie Van Leeuwenhoek. 1972;38(1):33–47. doi: 10.1007/BF02328075. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. The isolation of intracellular membranes of Escherichia coli 0111a. Methods Enzymol. 1974;31:633–642. doi: 10.1016/0076-6879(74)31069-5. [DOI] [PubMed] [Google Scholar]
- Hasin M., Rottem S., Razin S. The outer membrane of Proteus mirabilis. I. Isolation and characterization of the outer and cytoplasmic membrane fractions. Biochim Biophys Acta. 1975 Feb 14;375(3):381–394. doi: 10.1016/0005-2736(75)90354-5. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. S., Finnerty W. R. Microbial assimilation of hydrocarbons. I. The fine-structure of a hydrocarbon oxidizing Acinetobacter sp. Arch Microbiol. 1975;102(2):75–83. doi: 10.1007/BF00428349. [DOI] [PubMed] [Google Scholar]
- Kennedy R. S., Finnerty W. R. Microbial assimilation of hydrocarbons. II. Intracytoplasmic membrane induction in Acinetobacter sp. Arch Microbiol. 1975;102(2):85–90. doi: 10.1007/BF00428350. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Microbial assimilation of hydrocarbons: identification of phospholipids. J Bacteriol. 1970 Aug;103(2):348–355. doi: 10.1128/jb.103.2.348-355.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A., Lockwood P. J., Finnerty W. R. Comparative analysis of the lipids of Acinetobacter species grown on hexadecane. J Bacteriol. 1975 Jan;121(1):250–258. doi: 10.1128/jb.121.1.250-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Nieth K. F., Drews G. The protein patterns of intracytoplasmic membranes and reaction center particles isolated from Rhodopseudomonas capsulata. Arch Mikrobiol. 1974 Mar 4;96(2):161–174. doi: 10.1007/BF00590173. [DOI] [PubMed] [Google Scholar]
- Oelze J., Drews G. Membranes of photosynthetic bacteria. Biochim Biophys Acta. 1972 Apr 18;265(2):209–239. doi: 10.1016/0304-4157(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Oltmann L. F., Stouthamer A. H. Purification of cytoplasmic membranes and outer membranes from Proteus mirabilis. Arch Mikrobiol. 1973 Nov 19;93(4):311–325. doi: 10.1007/BF00427928. [DOI] [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pate J. L., Shah V. K., Brill W. J. Internal membrane control in Azotobacter vinelandii. J Bacteriol. 1973 Jun;114(3):1346–1350. doi: 10.1128/jb.114.3.1346-1350.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch V. M., Jr, Burger M. M. The bacterial mesosome. Biochim Biophys Acta. 1973 Apr 3;300(1):79–104. doi: 10.1016/0304-4157(73)90012-9. [DOI] [PubMed] [Google Scholar]
- Rogers S. W., Gilleland H. E., Jr, Eagon R. G. Characterization of a protein-lipopolysaccharide complex released from cell walls of Pseudomonas aeruginosa by ethylenediaminetetraacetic acid. Can J Microbiol. 1969 Jul;15(7):743–748. doi: 10.1139/m69-130. [DOI] [PubMed] [Google Scholar]
- STEWART J. E., KALLIO R. E., STEVENSON D. P., JONES A. C., SCHISSLER D. O. Bacterial hydrocarbon oxidation. I. Oxidation of n-hexadecane by a gram-negative coccus. J Bacteriol. 1959 Sep;78:441–448. doi: 10.1128/jb.78.3.441-448.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Bacterial membranes. CRC Crit Rev Microbiol. 1971 May;1(1):161–197. doi: 10.3109/10408417109104480. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. C., Finnerty W. R. Characterization of intracytoplasmic hydrocarbon inclusions from the hydrocarbon-oxidizing Acinetobacter species HO1-N. J Bacteriol. 1976 Jul;127(1):481–489. doi: 10.1128/jb.127.1.481-489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Glauert A. M. Chemical analysis of the outer membrane and other layers of the cell envelope of Acinetobacter sp. J Bacteriol. 1973 Oct;116(1):410–417. doi: 10.1128/jb.116.1.410-417.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M., Sleytr U. B. Isolation of outer membranes with an ordered array of surface subunits from Acinetobacter. J Bacteriol. 1973 Jun;114(3):1294–1308. doi: 10.1128/jb.114.3.1294-1308.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wallace W., Nicholas D. J. The biochemistry of nitrifying microorganisms. Biol Rev Camb Philos Soc. 1969 Jul;44(3):359–391. doi: 10.1111/j.1469-185x.1969.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Weigand R. A., Holt S. C., Shively J. M., Decker G. L., Greenawalt J. W. Ultrastructural properties of the extra membranes of Escherichia coli O111a as revealed by freeze-fracturing and negative-staining techniques. J Bacteriol. 1973 Jan;113(1):433–444. doi: 10.1128/jb.113.1.433-444.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Lennarz W. J., Schnaitman C. A. Distribution of lipids in the wall and cytoplasmic membrane subfractions of the cell envelope of Escherichia coli. J Bacteriol. 1972 Feb;109(2):686–690. doi: 10.1128/jb.109.2.686-690.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]