Abstract
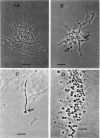
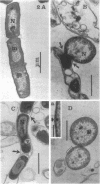
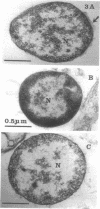
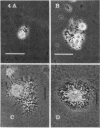
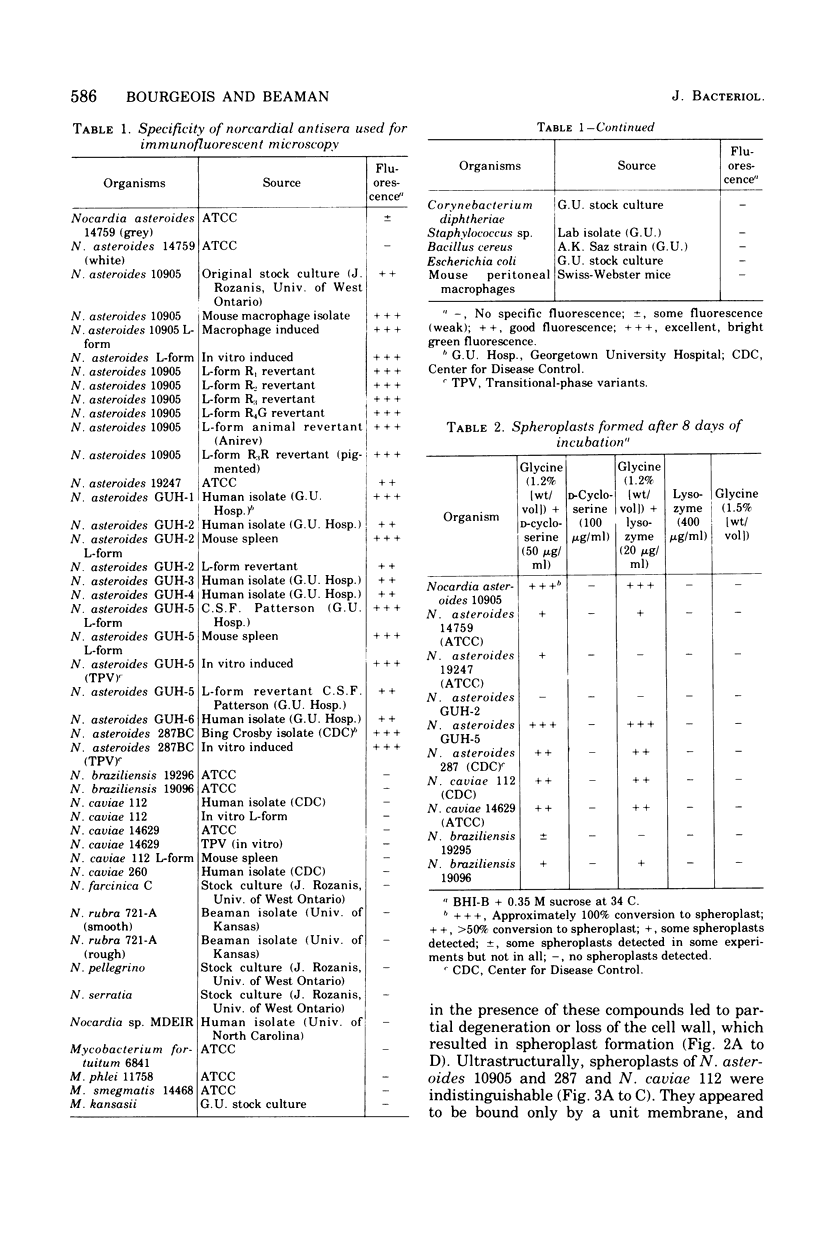
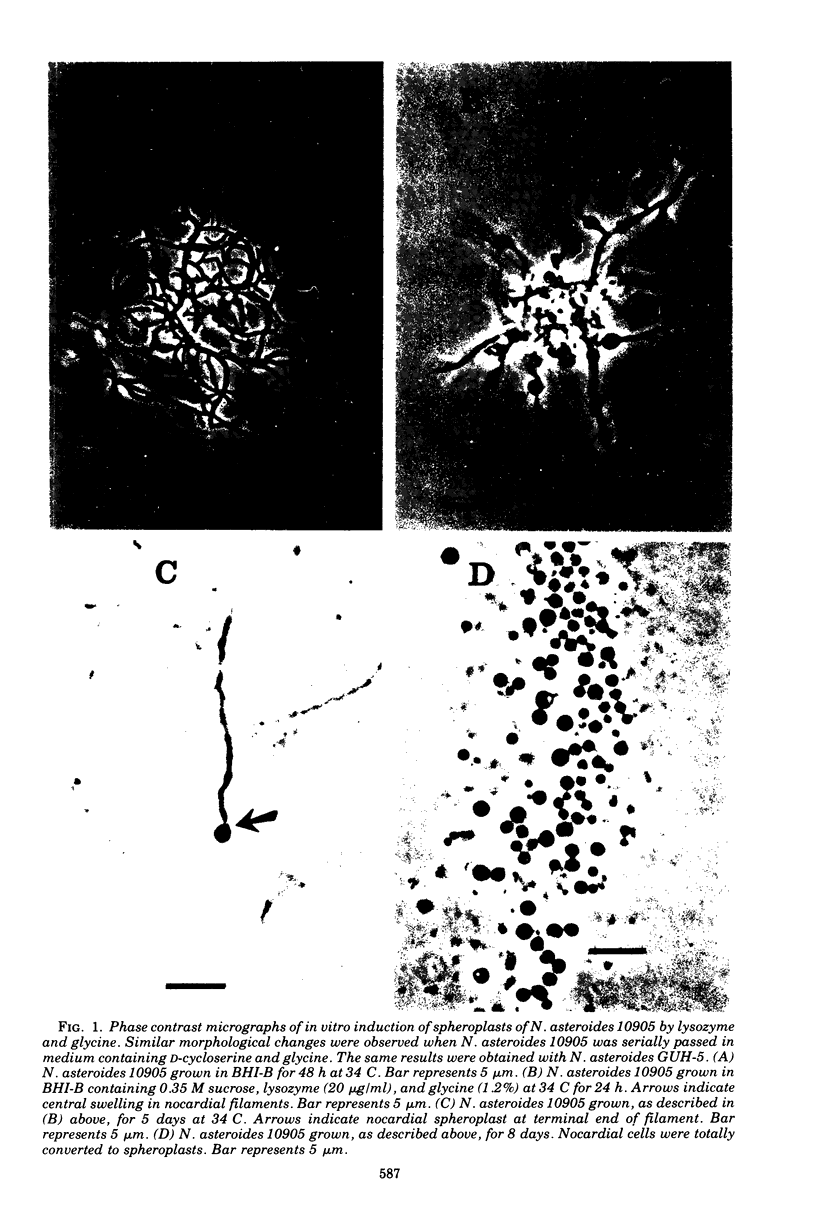
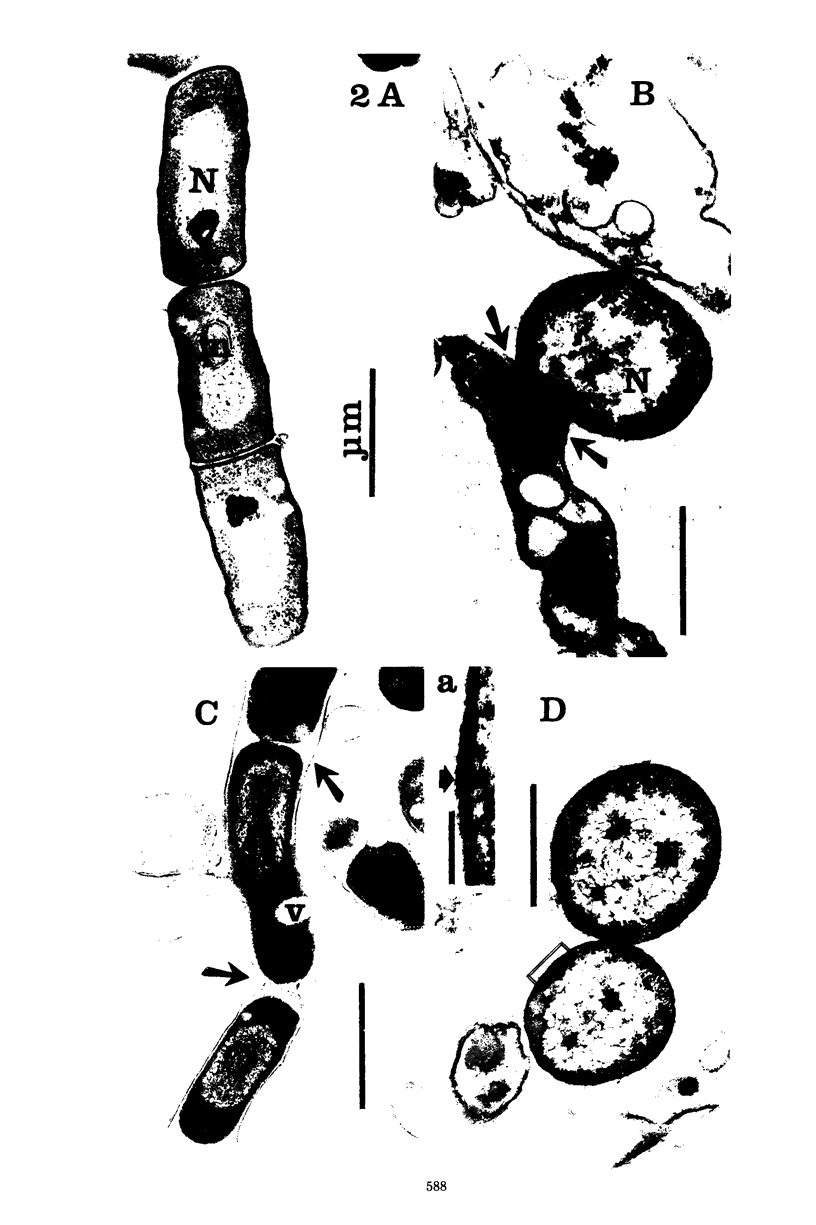
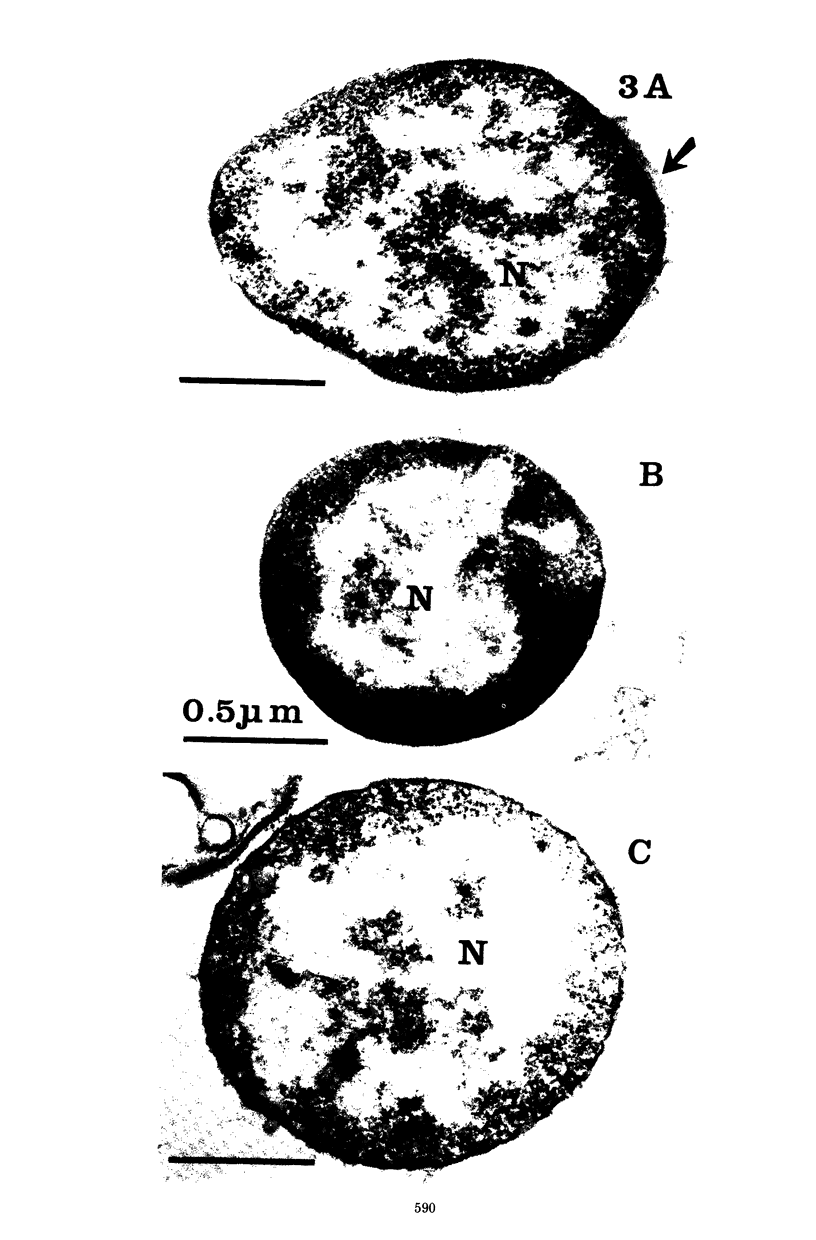
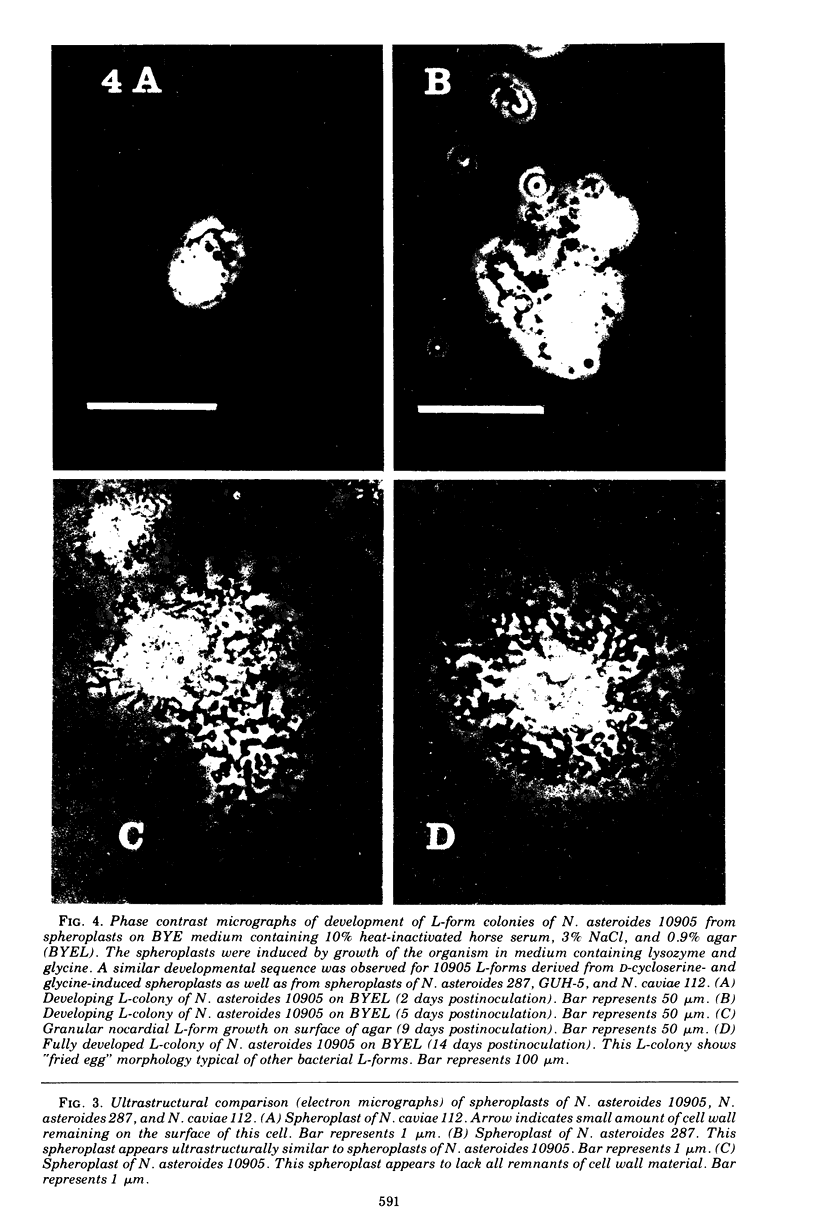
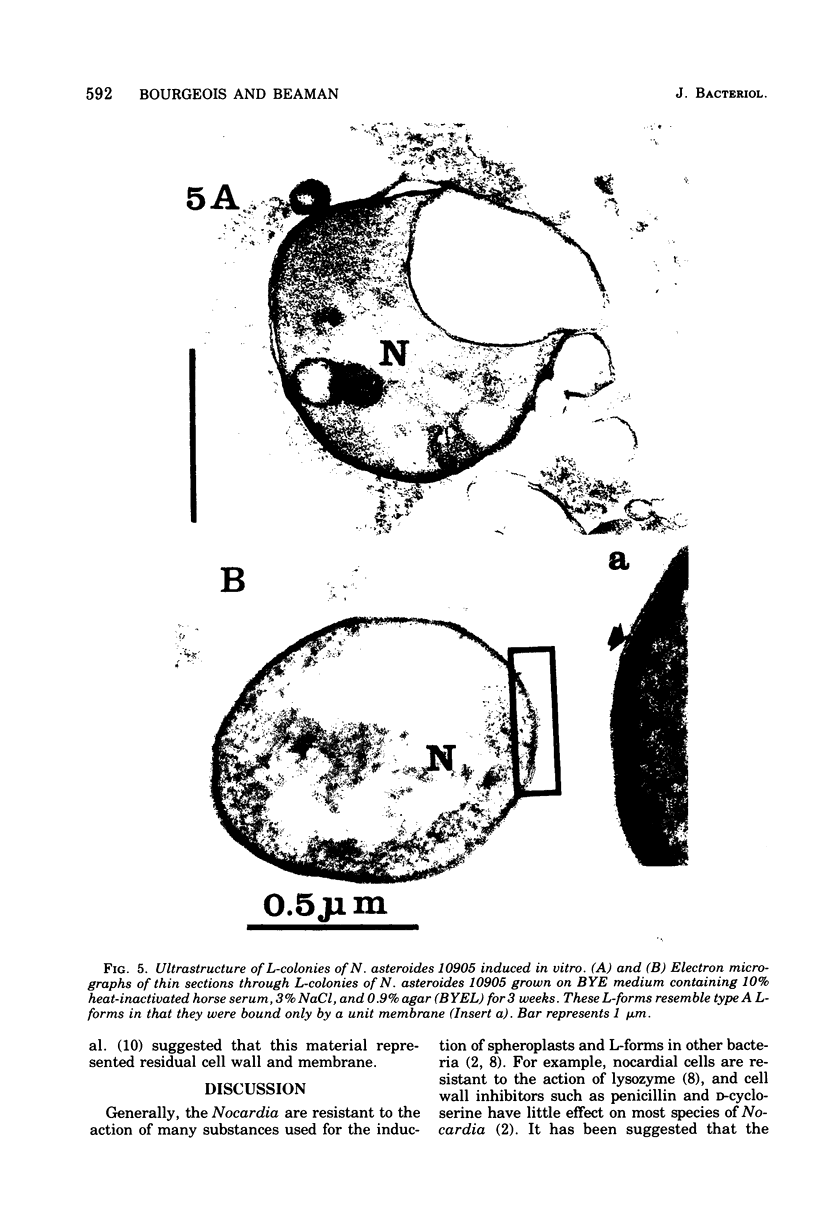
Six strains of Nocardia asteroides, two strains of N. caviae, and two strains of N. braziliensis were grown in medium supplementted with glycine, lysozyme, D-cycloserine, glycine plus lysozyme, and glycine plus D-cycloserine. It was shown that three strains of N. asteroides, and two strains of N. caviae, readily formed spheroplasts and/or protoplasts when grown in the presence of glycine plus either lysozyme or D-cycloserine. This process was studied by both phase contrast microscopy and electron microscopy. The induced cultures were then plated on hypertonic medium for the isolation of L-forms. It was shown that the organisms differed greatly in their ability to produce spheroplasts and subsequently grew as L-forms or transitional-phase variants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adámek L., Mison P., Mohelská H., Trnka L. Ultrastructural organization of spheroplasts induced in Mycobacterium sp. smegmatis by lysozyme or glycine. Arch Mikrobiol. 1969;69(3):227–236. doi: 10.1007/BF00408975. [DOI] [PubMed] [Google Scholar]
- BARILE M. F., YAGUCHI R., EVELAND W. C. A simplified medium for the cultivation of pleuropneumonia-like organisms and the L-forms of bacteria. Am J Clin Pathol. 1958 Aug;30(2):171–176. doi: 10.1093/ajcp/30.2_ts.171. [DOI] [PubMed] [Google Scholar]
- Bach M. C., Sabath L. D., Finland M. Susceptibility of Nocardia asteroides to 45 antimicrobial agents in vitro. Antimicrob Agents Chemother. 1973 Jan;3(1):1–8. doi: 10.1128/aac.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Kim K. S., Salton M. R., Barksdale L. Amino acids of the cell wall of Nocardia rubra. J Bacteriol. 1971 Nov;108(2):941–943. doi: 10.1128/jb.108.2.941-943.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Structural and biochemical alterations of Nocardia asteroides cell walls during its growth cycle. J Bacteriol. 1975 Sep;123(3):1235–1253. doi: 10.1128/jb.123.3.1235-1253.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois L., Beaman B. L. Probable L-forms of Nocardia asteroides induced in cultured mouse peritoneal macrophages. Infect Immun. 1974 Mar;9(3):576–590. doi: 10.1128/iai.9.3.576-590.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross T., Goodfellow M. Taxonomy and classification of the actinomycetes. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:11–112. [PubMed] [Google Scholar]
- DIENES L., WEINBERGER H. J. The L forms of bacteria. Bacteriol Rev. 1951 Dec;15(4):245–288. doi: 10.1128/br.15.4.245-288.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Carleton J., Watanakunakorn C., Klainer A. S., Hamburger M. Scanning-beam electron microscopy of cell wall-defective staphylococci. Infect Immun. 1970 Oct;2(4):504–515. doi: 10.1128/iai.2.4.504-515.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes W., Schleifer K. H., Kandler O. Mode of action of glycine on the biosynthesis of peptidoglycan. J Bacteriol. 1973 Nov;116(2):1029–1053. doi: 10.1128/jb.116.2.1029-1053.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T., Kanetsuna F., Rieber M. In vitro effect of cycloserine on mycobacterial ultrastructure. Tubercle. 1968 Dec;49(4):385–396. doi: 10.1016/s0041-3879(68)80019-4. [DOI] [PubMed] [Google Scholar]
- Sato H., Diena B. B., Greenberg L. The production of spheroplasts by rapid-growing non-virulent mycobacteria. Can J Microbiol. 1965 Oct;11(5):807–810. doi: 10.1139/m65-109. [DOI] [PubMed] [Google Scholar]