Abstract
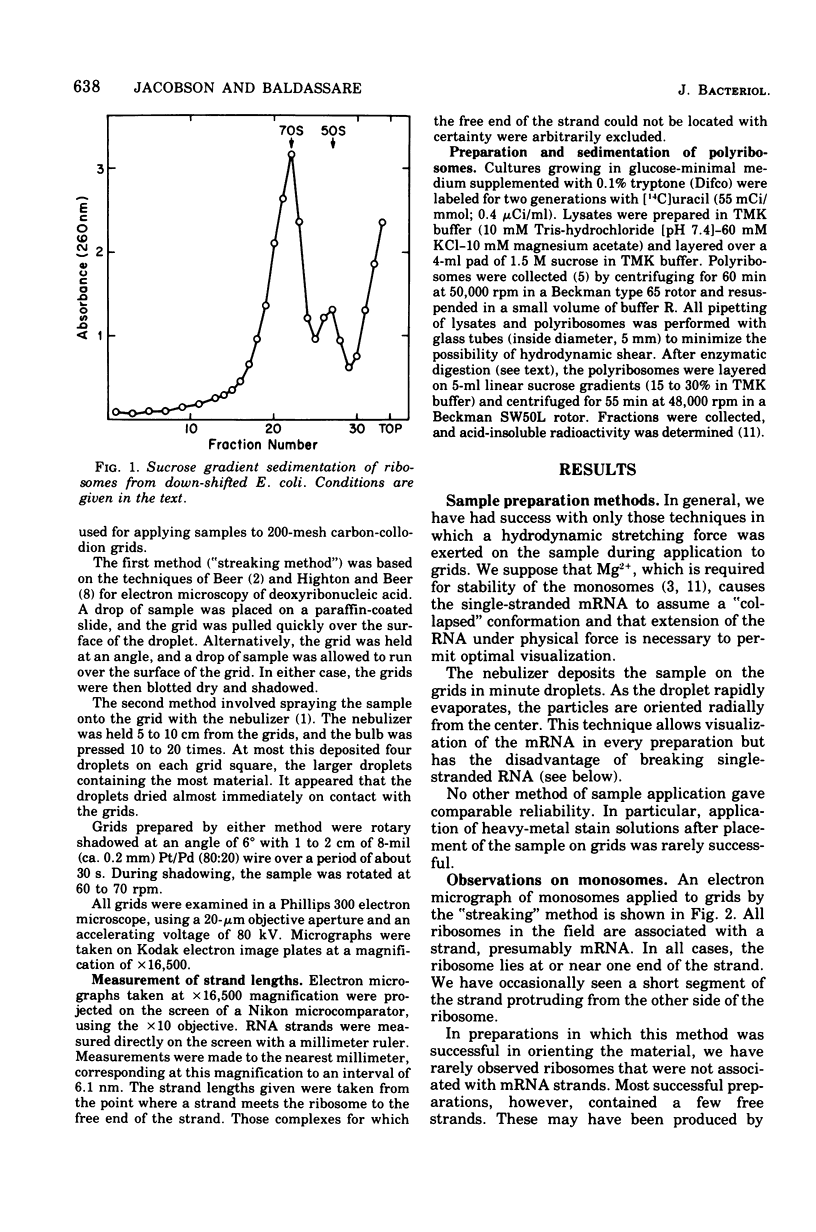
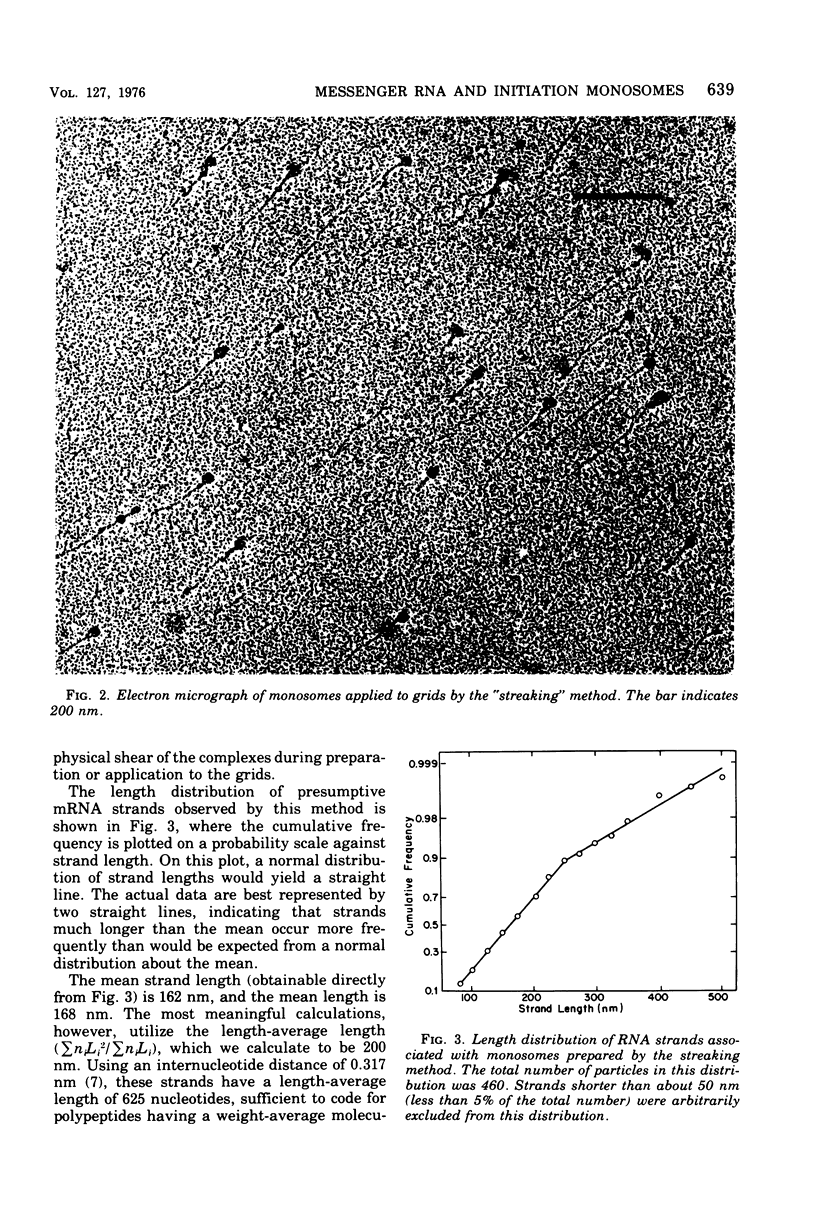
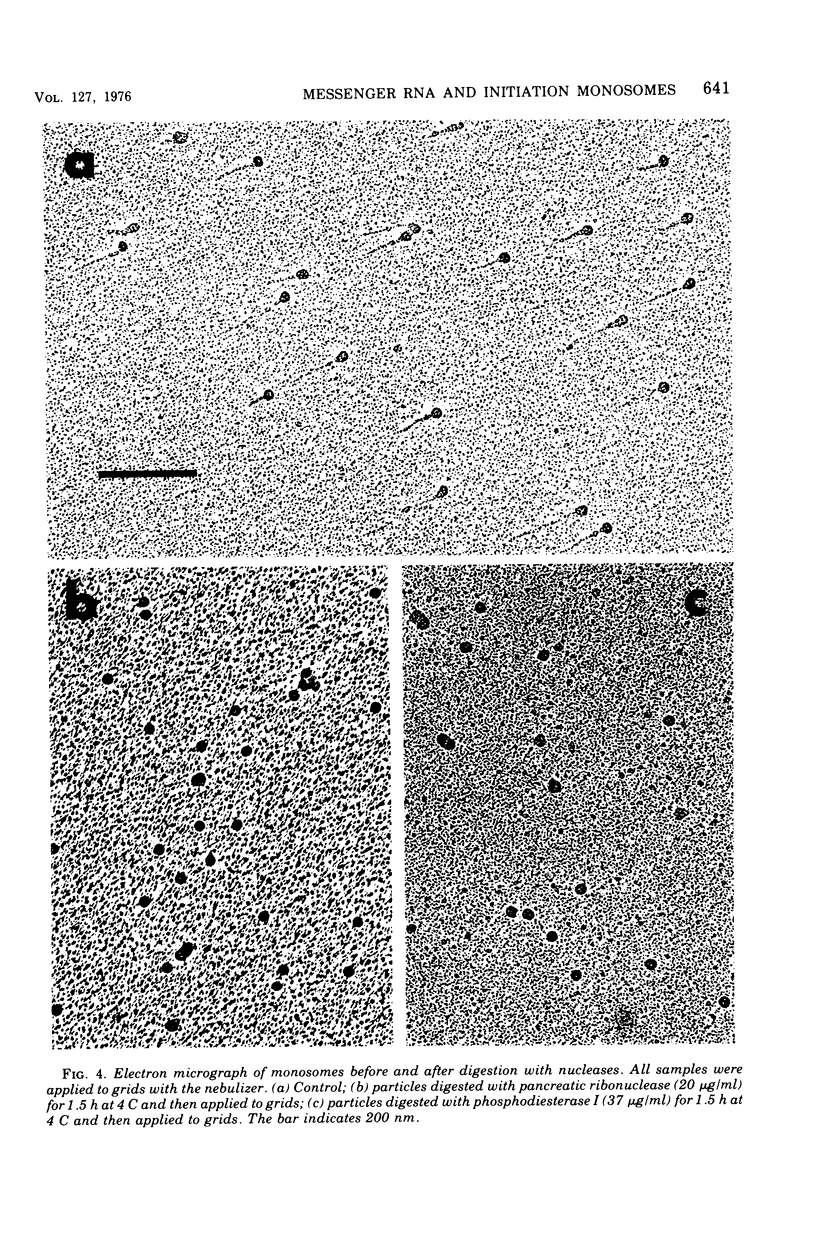
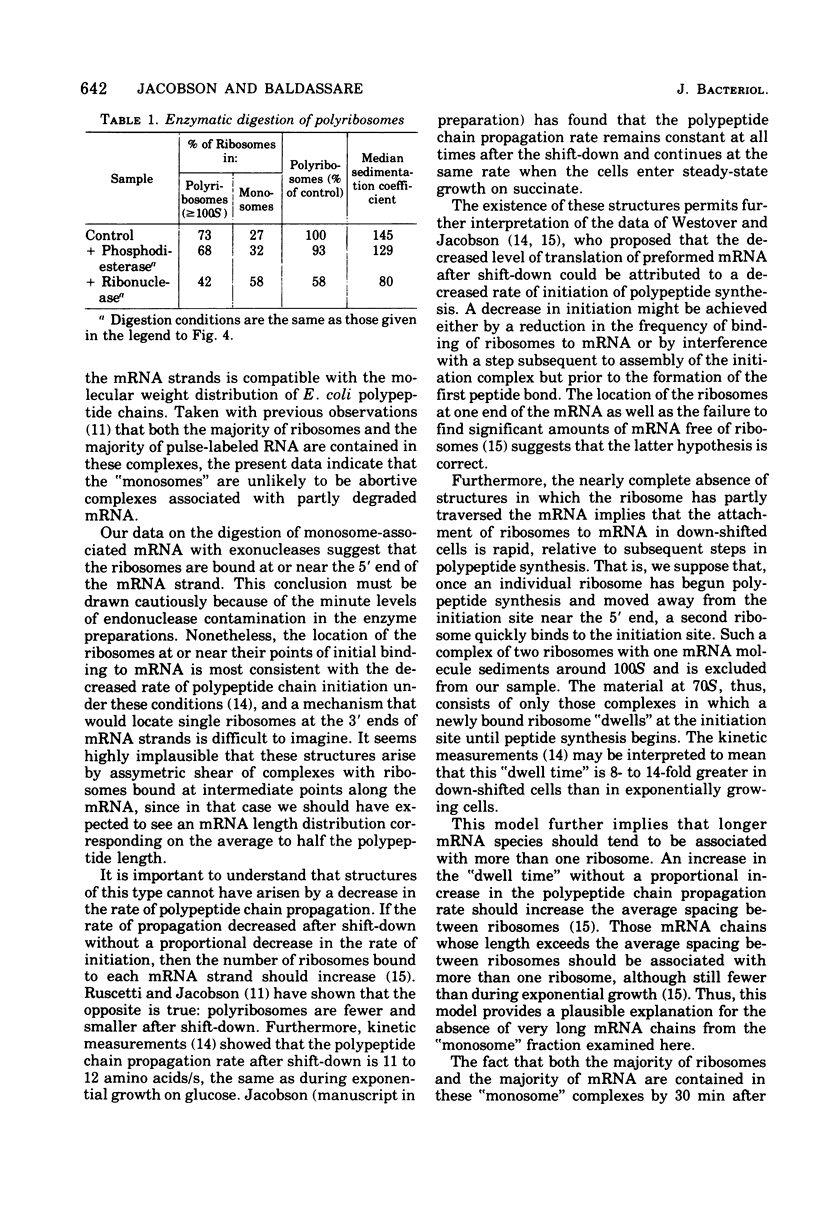
The complexed 70S ribosomes (monosomes) that accumulate in Escherichia coli after an energy source shift-down were examined in an electron microscope. In all cases, the ribosomes lie at or near one end of a ribonucleic acid (RNA) strand. This messenger RNA (mRNA) has a mean length of 168 nm and a length-average length of 200 nm, sufficient to code for polypeptides of a weight-average molecular weight of 20,000. The length distribution indicates that these strands are a reasonable representation of the population of monocistronic mRNA's of E. coli. The mRNA strands disappear entirely upon digestion with pancreatic ribonuclease, phosphodiesterase I, or polynucleotide phosphorylase. The susceptibility to digestion by 3'-exonucleases indicate that the ribosomes lie at the 5' end of the mRNA strands. These results are consistent with the hypothesis that down-shifted cells have a translational defect at a point subsequent to the binding of ribosomes to mRNA but prior to the formation of the first peptide bond, such that ribosomes remain bound at or near their points of initial attachment to mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER M. Electron microscopy of unbroken DNA molecules. J Mol Biol. 1961 Jun;3:263–266. doi: 10.1016/s0022-2836(61)80067-3. [DOI] [PubMed] [Google Scholar]
- Beller R. J., Davis B. D. Selective dissociation of free ribosomes of Escherichia coli by sodium ions. J Mol Biol. 1971 Feb 14;55(3):477–485. doi: 10.1016/0022-2836(71)90331-7. [DOI] [PubMed] [Google Scholar]
- Castles J. J., Singer M. F. Degradation of polyuridylic acid by ribonuclease II: protection by ribosomes. J Mol Biol. 1969 Feb 28;40(1):1–17. doi: 10.1016/0022-2836(69)90292-7. [DOI] [PubMed] [Google Scholar]
- Davies E., Larkins B. A. Polyribosome degradation as a sensitive assay for endolytic messenger-ribonuclease activity. Anal Biochem. 1974 Sep;61(1):155–164. doi: 10.1016/0003-2697(74)90342-x. [DOI] [PubMed] [Google Scholar]
- Friesen J. D. Control of messenger RNA synthesis and decay in Escherichia coli. J Mol Biol. 1966 Oct;20(3):559–573. doi: 10.1016/0022-2836(66)90011-8. [DOI] [PubMed] [Google Scholar]
- Granboulan N., Scherrer K. Visualisation in the electron microscope and size of RNA from animal cells. Eur J Biochem. 1969 May 1;9(1):1–20. doi: 10.1111/j.1432-1033.1969.tb00569.x. [DOI] [PubMed] [Google Scholar]
- HIGHTON P. J., BEER M. An electromicroscopic study of extended single polynucleotide chains. J Mol Biol. 1963 Jul;7:70–77. doi: 10.1016/s0022-2836(63)80019-4. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. Initiation, elongation and inactivation of lac messenger RNA in Escherichia coli studied studied by measurement of its beta-galactosidase synthesizing capacity in vivo. J Mol Biol. 1971 Sep 28;60(3):453–472. doi: 10.1016/0022-2836(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Ron E. Z., Kohler R. E., Davis B. D. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science. 1966 Sep 2;153(3740):1119–1120. doi: 10.1126/science.153.3740.1119. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Jacobson L. A. Accumulation of 70S monoribosomes in Escherichia coli after energy source shift-down. J Bacteriol. 1972 Jul;111(1):142–151. doi: 10.1128/jb.111.1.142-151.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westover K. C., Jacobson L. A. Control of protein synthesis in Escherichia coli. II. Translation and degradation of lactose operon messenger ribonucleic acid after energy source shift-down. J Biol Chem. 1974 Oct 10;249(19):6280–6287. [PubMed] [Google Scholar]
- Westover K. C., Jacobson L. A. Control of protein synthesis of Escherichia coli. I. Translation and functional inactivation of messenger ribonucleic acid after energy source shift-down. J Biol Chem. 1974 Oct 10;249(19):6272–6279. [PubMed] [Google Scholar]




