Abstract
We previously reported the presence of a novel variant (β-T594M) of the amiloride-sensitive Na+ channel (ASSC) in which the threonine residue at position 594 in the β-subunit has been replaced by a methionine residue. Electrophysiological studies of the ASSC on Epstein–Barr virus (EBV)-transformed lymphocytes carrying this variant showed that the 8-(4-chlorophenylthio) adenosine 3′:5′-cyclic monophosphate (8cpt-cAMP)-induced responses were enhanced when compared to wild-type EBV-transformed lymphocytes. Furthermore, in wild-type EBV-transformed cells, the 8cpt-cAMP-induced response was totally blocked by the phorbol ester, phorbol 12-myristate 13-acetate (PMA). This inhibitory effect of PMA was blocked by a protein kinase C inhibitor, chelerythrine. We now have identified individuals who are homozygous for this variant, and showed that PMA had no effect on the 8cpt-cAMP-induced responses in the EBV-transformed lymphocytes from such individuals. Cells heterozygous for this variant showed mixed responses to PMA, with the majority of cells partially inhibited by PMA. Our results demonstrate that an alteration in a single amino acid residue in the β-subunit of the ASSC can lead to a total loss of inhibition to PMA, and establish the β-subunit as having an important role in conferring a regulatory effect on the ASSC of lymphocytes.
The amiloride-sensitive Na+ channel (ASSC) or epithelial sodium channel (ENaC) plays a crucial role in the maintenance of sodium balance, extracellular fluid volume, and blood pressure. Recently, the ASSC has been cloned and characterized (we use ASSC instead of ENaC because (i) lymphocytes are not epithelial in nature or origin, (ii) though the human β-subunit of ENaC is expressed in lymphocytes (1), the α-subunit of this channel is reported to be different from that of rat or human ENaC (see discussion in ref. 2), and (iii) the gating characteristics of the lymphocyte ASSC channel is different from that of the expressed ENaC (R.Y.K.P., personal observation). It is comprised of at least three subunits: an α-, β-, and γ-subunit. The α-subunit appears to be the conducting unit because expression of the subunit by itself in Xenopus oocytes results in low levels of amiloride-sensitive Na+ current. The role of the other two subunits is less certain. Neither the β- nor γ-subunit, when expressed alone or together, produced any measurable Na+ current. However, coexpression with the α-subunit greatly enhanced the amplitudes of the Na+ current (3, 4). In addition, truncation of the carboxyl terminus of either the β or γ, as found in a rare autosomal dominant disease known as Liddle syndrome (4–7), leads to an increase in basal activity and a reduction in turnover of the ASSC (8–10). Modification of the Gly residues near the second transmembrane domain of the β- and γ-subunits alters the binding affinity to amiloride and the conductance of the channel (11). These results indicate that the β- and γ-subunits probably have a structural and/or regulatory role in the stabilization and function of the channel.
We recently identified a novel variant in the β-subunit of the ASSC in which the amino acid residue threonine at position 594 is changed to a methionine (β-T594M; ref. 12). Whole-cell voltage clamp recordings of lymphocytes from patients with the variant channel showed a greater response following stimulation with 8-(4-chlorophenylthio) adenosine 3′:5′-cyclic monophosphate (8cpt-cAMP), a membrane permeant analog of cAMP when compared to wild-type lymphocytes. We proposed that the enhanced cAMP response might result from a loss of inhibition of the channel. Because the Thr residue has been proposed to be a consensus site for protein kinase C (PKC) phosphorylation (13), we investigated the effects of the phorbol ester phorbol 12-myristate 13-acetate (PMA), which activates PKC, on Epstein–Barr virus (EBV)-transformed lymphocytes with or without this variant channel. We found that the 8cpt-cAMP-induced responses in wild-type EBV-transformed cells were completely blocked by PMA, whereas in cells which are homozygous for this variant, PMA had no effect on the 8cpt-cAMP-induced responses. Because the inhibitory effect of PMA could be blocked by a PKC-antagonist chelerythrine, our results show that the PKC-mediated negative regulation of the homozygous β-T594M variant is absent.
EXPERIMENTAL PROCEDURES
In Vitro Transformation of Lymphocytes by EBV.
Lymphocytes were isolated on a Ficoll gradient from fresh blood samples obtained from individuals carrying either the β-T594M variant or not (wild type). The cells were transformed by EBV virus using a standard procedure with cyclosporin. The EBV-transformed cell lines were maintained in culture in RPMI 1640 medium with 20% fetal bovine serum and antibiotics (100 units of ampicillin and streptomycin).
Genotype Analysis.
DNA was extracted from EBV-transformed lymphocytes using a protocol and reagents from the Puregene DNA isolation kit (Gentra Systems, Minneapolis). PCR was performed using primers and conditions described previously (12). After PCR, 15 μl of the PCR was digested with 20 units of NlaIII (New England Biolabs) for 2 hr and the products were analyzed on a 1.5% agarose gel.
Identification of the T594M Variant from EBV-Transformed Lymphocytes.
Total RNA was isolated from EBV-transformed lymphocytes using Tri Reagent (Molecular Research Center, Cincinnati). First strand cDNA was synthesized from the isolated total RNA using the SuperScript Preamplification System (GIBCO/BRL). After reverse transcription, PCR was performed using the same set of primers that were used for genotyping with the following conditions: 2 μl of first strand cDNA, 6 pmol of each primer, 5 μl of 5× PCR buffer B (Invitrogen), 50 pmol each of dATP, dCTP, dGTP, dTTP, 3 μl of dimethyl sulfoxide, and 0.5 unit of Taq DNA polymerase (GIBCO/BRL) in a total volume of 25 μl. Thermocycling conditions were initial denaturation for 5 min at 94°C, followed by 35 cycles of 58°C for 45 sec, 72°C for 45 sec, and 94°C for 45 sec. NlaIII digestion was carried out following reverse transcription–PCR, and the products were analyzed on a 1.5% agarose gel.
Electrophysiological Studies.
Whole-cell voltage clamp experiments were performed under either the perforated-patch or the whole-cell tight-seal mode with an Axopatch 1-A amplifier (Axon Instruments, Foster City, CA). Perforated-patch recordings were performed as described (12). These experiments were performed to establish that the EBV-transformed cells had similar membrane responses to freshly isolated lymphocytes. Briefly, bath medium was RPMI 1640 containing low Ca2+ (0.54 mM) supplemented with 15 mM Hepes to buffer the pH. The pipet solution contained 70 mM KCl, 30 mM K2SO4, 12 mM NaCl, 0.5 mM EGTA, 1 mM MgCl2, 20 mM Hepes, and 10 mM glucose; pH was adjusted to 7.2 and osmolarity to 300 mOsm. Whole-cell tight-seal recordings, in which the membrane beneath the pipet tip was ruptured by further suction, were made when evaluating the modulating effects of PKC using PMA. In contrast to the perforated-patch experiments where 8cpt-cAMP was applied extracellularly via a perfusion pipet placed nearby, 8cpt-cAMP was applied intracellularly by including the drug in the pipet solution for the experiments using PMA. The recording solution was similar to that used in perforated-patch recordings with 60 mM KCl replacing the K2SO4. To evaluate whether the effects of PMA were mediated via PKC, chelerythrine (5 μM; LC Laboratories, Woburn, MA), a PKC-selective antagonist (14), was included in the pipet solution with the 8cpt-cAMP.
Membrane currents were elicited by a voltage ramp from −150 to +50 mV of 1 sec duration. Four ramps at 0.2 Hz were averaged for each measurement. Execution of the ramp and collection of data were controlled by a computer with the software pclamp (version 5.5; Axon Instruments). Current records were filtered at 5 kHz, digitized, and stored for later analysis. We measured the slope of the linear portion of the current (or the best linear line through the current trace) between −120 and −50 mV and used it as the measurement of the responses. Because the recorded cells had similar membrane capacitance [wild type, 6.59 ± 1.30 pF (n = 54); heterozygous, 6.50 ± 1.39 pF (n = 51); homozygous, 6.82 ± 1.63 pF (n = 51)], we did not normalize the slope conductance to the membrane capacitance and used the measured slope conductance values for direct comparisons. All reported parameters were expressed as mean ± SD and statistical comparisons were made with ANOVA.
We chose a concentration of 300 μM 8cpt-cAMP for our studies. This concentration was effective in enhancing ASSC activity (15, 16), and further allowed us to compare the present results to those obtained previously (12). This concentration produces a maximal increase in slope conductance (higher concentrations did not produce a further increase), which would rule out the possibility of any nonphosphorylated channels that might affect the results of the PKC studies. A total of 100 μM 8cpt-cAMP produced a similar increase in slope conductance, but the time it took for stable responses to be attained was variable. The concentration of PMA used in our studies was 200 nM.
RESULTS
Genotype: Wild Type, Heterozygous, and Homozygous for T594M Variant.
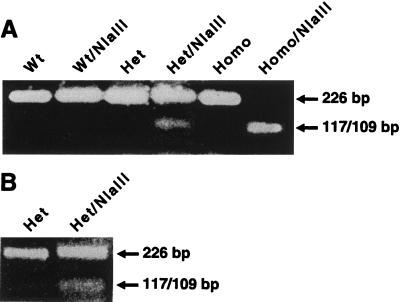
The single nucleotide sequence substitution of T for C in the β-T594M variant creates a unique NlaIII restriction enzyme site. The creation of this site meant that the 226-bp PCR product can be cleaved into two fragments, a 117 bp and a 109 bp, which comigrate on a 1.5% agarose gel. NlaIII digestion of PCR products using genomic DNA from wild-type cells gave a single uncut 226-bp fragment. NlaIII digestion of PCR products from cells homozygous for the β-T594M variant gave only the 117- and 109-bp fragments, whereas cells heterozygous for the β-T594M variant showed both the uncut wild-type 226-bp fragment and the 117/109-bp variant fragments (Fig. 1A). Reverse transcription–PCR products from the EBV-transformed lymphocytes digested by NlaIII showed a similar pattern (Fig. 1B).
Figure 1.
Genotype analysis of T594M variant in the β-subunit of ASSC. (A) A 1.5% agarose gel showing products using genomic DNA as template from cells which are wild type (Wt), heterozygous (Het) for the T594M variant, or homozygous (Homo) for the T594M variant. The changing of nucleotide sequence C → T in the T594M variant generates a novel NlaIII site at this position. Also shown in A are the results of digesting each of these PCR products with the enzyme NlaIII. (B) A 1.5% agarose gel analysis of reverse transcription–PCR products from EBV-transformed lymphocytes heterozygous for T594M variant without (Het) and with (Het/NlaIII) enzyme digestion.
Comparison of Responses Between Lymphocytes and EBV-Transformed Cells.
We examined the effects of 8cpt-cAMP on the EBV-transformed lymphocytes and compared the results to those obtained previously from lymphocytes freshly isolated from peripheral blood. The data, shown in Table 1, indicate that there is no significant difference in the 8cpt-cAMP-induced increase in slope conductance between freshly isolated lymphocytes and the EBV-transformed lymphocytes. Similar to the findings in the freshly isolated lymphocytes, the enhanced responses to 8cpt-cAMP of EBV-transformed cells are also blocked by amiloride (Table 1).
Table 1.
Comparison of the responses to 8cpt-cAMP between lymphocytes and EBV-transformed cells
| Basal, nS | 8cpt-cAMP, nS | 8cpt-cAMP + amiloride, nS | |
|---|---|---|---|
| Wild type | |||
| Lymphocyte | 0.43 ± 0.24 (15) | 1.20 ± 0.72* (15) | 0.36 ± 0.24 (10) |
| EBV-transformed cell | 0.43 ± 0.07 (8) | 1.32 ± 0.24* (8) | 0.50 ± 0.15 (4) |
| β-T594M variant | |||
| Lymphocyte (overall) | 0.49 ± 0.26 (13) | 2.07 ± 1.19* (13) | 0.57 ± 0.30 (7) |
| Wild-type-like (<2nS, 7) | 0.45 ± 0.28 | 1.23 ± 0.47* | |
| “Intermediate” (2-4 nS; 5) | 0.52 ± 0.24 | 2.68 ± 0.51* | |
| Homozygous-like (>4 nS, 1) | 0.75 | 4.87 | |
| EBV-transformed cell | 0.43 ± 0.10 (8) | 2.30 ± 0.52* (8) | 0.44 ± 0.14 (8) |
Data on the variant lymphocytes are recalculated values based on the results from ref. 12. Similar to the findings for the EBV-transformed cells heterozygous for the variant, three groups of responses can be distinguished based on the increase in slope conductance to cAMP. Numbers in parentheses are the number of cells studied. The values for cAMP are significantly different from their basal values (∗, P > 0.05). The values for 8cpt-cAMP plus amiloride are not different from the basal values. The value for the homozygous-like cell was not tested.
Effects of PMA on 8cpt-cAMP-Induced Responses in EBV-Transformed Cells.
Only cells in which stable and consistent responses were recorded and where recovery from drug studies could be obtained were included in the final data analysis. Basal conductance was measured within 10 sec of obtaining whole-cell tight-seal recording (as indicated by the appearance of capacitive transients). The 8cpt-cAMP-induced increase in slope conductance on both wild-type cells and cells heterozygous for the variant (Table 2) with intracellular application are not significantly different from those obtained with extracellular superfusion (Table 1), though more cells appear to respond to 8cpt-cAMP when it was applied intracellularly (70% of 156 cells) compared to when it was applied extracellularly (42% of 104 cells).
Table 2.
Summary of the effects of PMA on 8cpt-cAMP-induced responses in EBV-transformed lymphocytes
| Basal, nS | 8cpt-cAMP, nS | 8cpt-cAMP + PMA, nS | |
|---|---|---|---|
| Wild-type | |||
| Absence of chelerythrine | 0.40 ± 0.14 (12) | 1.42 ± 0.59 (12) | 0.38 ± 0.16* (9) |
| With chelerythrine | 0.32 ± 0.20 (4) | 1.33 ± 0.41 (4) | 1.10 ± 0.44 (4) |
| Heterozygous (overall, 24) | 0.46 ± 0.14 | 3.03 ± 1.38 | 1.99 ± 1.65* |
| Wild-type-like (5) | 0.51 ± 0.18 | 1.42 ± 0.38 | 0.54 ± 0.19* |
| “Intermediate” (14) | 0.44 ± 0.13 | 2.93 ± 0.78 | 1.51 ± 0.64* |
| Homozygous-like (5) | 0.47 ± 0.07 | 4.92 ± 1.06 | 4.79 ± 1.04 |
| Homozygous (16) | 0.52 ± 0.17 | 4.71 ± 1.28 | 4.60 ± 1.27 |
In the wild-type group, the presence of chelerythrine in the pipet solution significantly reduced the inhibition by PMA (P < 0.001) but had no effect on either the basal conductance or the response to 8cpt-cAMP. The values obtained for PMA are significantly different from the slope conductance values obtained with 8cpt-cyclic AMP alone with the exception of the homozygous-like and homozygous variant: ∗, P < 0.05. 8cpt-cAMP was applied intracellularly via the recording pipet.
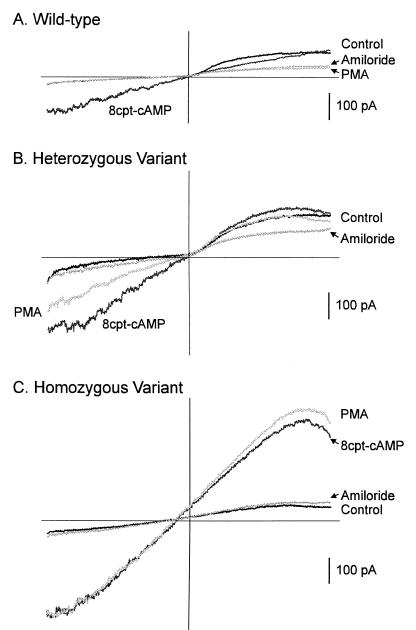
Once stable responses were recorded, PMA was applied from another pipet placed nearby. For the wild-type cells the increase in slope conductance to 8cpt-cAMP was completely blocked by PMA. An example of such an observation is shown in Fig. 2A. In EBV-transformed cells homozygous for the variant channels, 8cpt-cAMP induced an increase in slope conductance which was significantly higher than those measured for heterozygous and wild-type cells (Table 2). However, PMA had no effect on the 8cpt-cAMP-induced enhanced slope conductance (Fig. 2C and Table 2). Although PMA failed to affect the increase in slope conductance induced by 8cpt-cAMP, application of amiloride (2 μM) reversed this increase and brought it back to basal level (Fig. 2C). On the other hand, amiloride failed to decrease the PMA-suppressed response further in wild-type cells (Fig. 2A). The blockage by amiloride was reversible (data not shown).
Figure 2.
Membrane currents elicited by a voltage ramp from wild-type EBV-transformed cells (A), EBV-transformed cells heterozygous for the variant (B), and EBV-transformed cells homozygous for the variant (C). 8cpt-cAMP increased the slope of the current response for all three cells. The enhanced slope was totally blocked by PMA in the wild-type cell. Thereafter, addition of amiloride failed to change the slope further (A). In the cell heterozygous for the variant, PMA partially blocked the 8cpt-cAMP response. Further addition of amiloride abrogated the induced response (B). Although PMA had no effect on the cAMP-induced increase in the slope of the current response in the cell homozygous for the variant, further addition of amiloride blocked this increase and brought it back to the control level (C). Note that for the cell homozygous for the variant the increase in slope conductance by 8cpt-cAMP was larger.
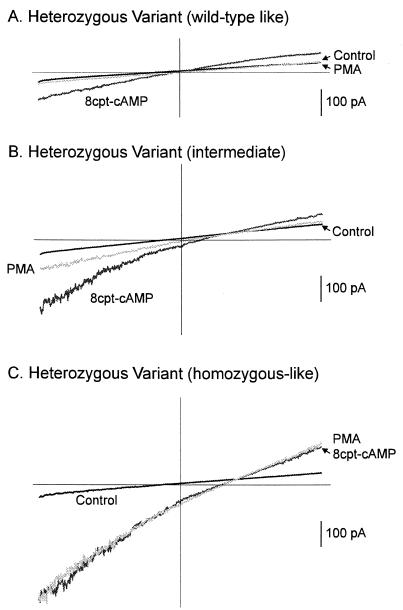
In cells heterozygous for the variant, PMA partially blocked the 8cpt-cAMP-induced response by about 50% and further application of amiloride reduced the slope conductance back to the basal level (Fig. 2B and Table 2). When individual cells were examined, three groups of responses could be distinguished. In the first group, responses similar to those of wild type were observed: 8cpt-cAMP enhanced the slope conductance to less than 2 nS and PMA blocked this increase completely (Fig. 3A and Table 2). In the second group, the cAMP-induced responses were intermediate (between 2–4 nS) and partially blocked by PMA (Fig. 3B and Table 2). In the third group, responses were similar to those of the cells homozygous for the variant: 8cpt-cAMP increased the slope conductance to >4 nS, but PMA had no effect on this increase (Fig. 3C and Table 2). When we re-examined the results obtained from the freshly isolated lymphocytes using the increase in slope conductance as an index, a similar pattern of responses could be found (Table 1).
Figure 3.
Membrane currents evoked by a voltage ramp from three EBV-transformed cells heterozygous for the variant. (A) A cell heterozygous for the variant that behaved like a wild-type cell—i.e., the increase in slope of the current response by 8cpt-cAMP could be blocked by PMA. (B) A cell in which the effect of cAMP was partially blocked by PMA. (C) A cell heterozygous for the variant that behaved like a homozygous cell. For this cell, PMA had no effect on the increase in the slope of the current response induced by 8cpt-cAMP.
To determine whether the inhibitory effects of PMA on the increase in slope conductance induced by 8cpt-cAMP were mediated via PKC, we studied the effects of chelerythrine (5 μM) by including this compound in the pipet solution together with 8cpt-cAMP on wild-type EBV-transformed cells. Chelerythrine had no effect on the basal slope conductance and did not alter the increase in slope conductance induced by 8cpt-cAMP. However, it significantly reduced the inhibitory action of PMA (Table 2).
DISCUSSION
We previously identified a novel variant of the β-subunit of ASSC in which the threonine at position 594 is changed to a methionine (β-T594M). We now have identified individuals who are heterozygous or homozygous for the β-T594M variant using genomic DNA. Moreover, the presence of this variant was verified in the EBV-transformed lymphocytes by reverse transcription–PCR. We hypothesized that the increase in membrane response to cAMP in lymphocytes with the variant channel was due to a loss of inhibition (12). To test this hypothesis we examined the membrane responses to 8cpt-cAMP in EBV-transformed lymphocytes that are wild-type, heterozygous, or homozygous for the variant respectively, and studied the effects of PMA, an activator of PKC, on the cAMP-induced responses. Our results showed that the β-T594M variant has lost its PKC-mediated inhibition. The results indicate that the β-subunit of the ASSC plays a critical role in conferring a negative regulatory site on the lymphocyte ASSC channel.
The effects of 8cpt-cAMP and amiloride on the EBV-transformed wild-type cells and cells heterozygous for the variant are comparable to those obtained for freshly isolated lymphocytes (Table 1). These results indicate that (i) EBV transformation has not altered the regulatory effects of cAMP nor the pharmacological properties of the ASSC, and (ii) the transformed cells do reflect the membrane characteristics of the freshly isolated lymphocytes. Thus, the even larger increase in slope conductance induced by cAMP observed in cells homozygous for the variant point to an intrinsic change in the properties of the variant channel.
This change is not related to the effects of cAMP on conductances other than ASSC. First, the slope conductances were measured between negative potentials below those which activated the outward conductances. Second, and more importantly, although PMA failed to reduce the cAMP-induced response in the cells homozygous for the variant, subsequent application of amiloride blocked the increase and reduced the slope conductance back to control levels, indicating that the enhanced response to cAMP in these cells was entirely mediated via the ASSC. The combined effects of PMA and amiloride unequivocally show that the 8cpt-cAMP-induced responses are solely due to the cAMP effects on the ASSC activated within the voltage range that we used in our measurements. Thus, the further increase in slope conductance induced by cAMP in the cells homozygous for the variant is likely to be related to an increase in the number of expressed channels, or perhaps an increase in efficacy of cAMP in enhancing the conductance. It is also possible that the probability of opening for the variant channel is greatly increased. Further experiments will be required to determine how the Thr → Met substitution leads to a larger increase in slope conductance, because this amino acid residue is outside the PY motif (see refs. 8–10).
The results of the effects of the phorbol ester PMA on the 8cpt-cAMP responses on the three groups of EBV-transformed cells (wild type, heterozygous variant, and homozygous variant) are summarized in Table 2. It can be seen that PMA abolished the cAMP-induced responses in wild-type EBV-transformed cells but had no effect on the cAMP-induced responses in cells homozygous for the variant. The effect of PMA is likely to be mediated via PKC because the inhibitory action of PMA was blocked in the presence of a selective PKC antagonist, chelerythrine. Though it is possible that the cells homozygous for the variant lack the enzyme PKC or that PMA somehow failed to activate PKC, this seems unlikely because PMA was effective on the majority of the EBV-transformed cells heterozygous for the variant. Whether the inhibition by PMA reported here is mediated via direct phosphorylation of the channel protein or indirectly via other proteins is not established in our studies. We favor the former because the Thr residue at position 594 has been proposed to be a consensus site for PKC (13), and mutation of this residue to a methionine residue in the variant led to a loss of inhibition to PKC. Bubien et al. (17) have proposed a model for ASSC based on their work on lymphocytes and EBV-transformed lymphocytes in which the modulatory effects of G-proteins and cAMP are dependent on the state of phosphorylation of the channel. Our data indicate that there are at least two separate, independent regulatory sites on the lymphocyte ASSC. One is acted on by cAMP and leads to an increase in channel activity; the other is acted on by PKC and results in a decrease in channel activity. Whether both sites are equally influenced by G-proteins will be a topic of future investigation. In addition to the β-subunit having a crucial role in mediating the turnover (9, 10), as well as amiloride binding and channel conductance (11) of the renal ASSC, we have established that the β-subunit also confers a negative regulatory site on the lymphocyte ASSC. In lipid bilayer studies, PKC has been reported to inhibit the activities of expressed bovine renal α-ENaC [in the absence of a β-subunit and cAMP (18)], thus raising the possibility that sites other than the Thr residue on the β-subunit can confer PKC inhibition on the renal ENaC.
Our results indicate that within the population of cells heterozygous for the variant (Table 2) there are cells that behave like wild type: cells in which the cAMP-induced increase in slope conductance is <2 nS and the increase is effectively blocked by PMA. There are cells that behave as if they are homozygous: cells in which the cAMP-induced response is >4 nS but PMA has no effect on this induced response. The majority of the cells have responses to cAMP that are intermediate between the wild type and cells homozygous for the variant. PMA partially blocked this induced response. Data from the lymphocytes isolated from patients showed a similar pattern of response (Table 1), though we did not examine the effects of PMA in that study. The underlying cause for this distribution is not clear.
Because the β-T594M variant is seen only in individuals of African descent, our results offer the intriguing prospect that variation in the ASSC may be a factor in the increased salt sensitivity prevalent in the African-American population. In Liddle syndrome, mutations of the carboxyl termini of the β- and/or γ-subunit of the ASSC protein lead to a reduction in the turnover of the channel. This apparent increase in activity could lead to an increased reabsorption of Na+, imbalance in salt and water retention, and possible increased blood pressure. The β-T594M does not appear to be a subset of Liddle syndrome for the following reasons. (i) The cAMP-induced response is enhanced in the β-T594M variant, whereas in Liddle syndrome, where the basal activity of the ASSC channel is increased, cAMP had no further potentiating effects (19). (ii) All mutations associated with Liddle appear to involve the proline-rich region of the β- or γ-subunit of the ASSC (8, 10), whereas the β-T594M variant is just outside of this PY-motif region. It is tempting to speculate that a loss of inhibitory regulation in the variant channel can lead to an increase in sensitivity to cAMP, and the resulting enhanced induction is a contributing factor in the predisposition to salt-sensitive hypertension in the African-American population.
Acknowledgments
We are grateful to Drs. Ken Blumenthal and Steve Kleene for their helpful comments. This work was supported in part by funds from the Markey Foundation, the National Institutes of Health Program of Excellence in Molecular Biology of Heart and Lung to A.G.M., and by a Cardiovascular Research Challenge Grant and Ohio Board of Regents Research Challenge Program to M.R., A.G.M., and R.Y.K.P.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ASSC, amiloride-sensitive Na+ channel; 8cpt-cAMP, 8-(4-chlorophenylthio) adenosine 3′:5′-cyclic monophosphate; PMA, phorbol 12-myristate 13-acetate; PKC, protein kinase C; EBV, Epstein–Barr virus; ENaC, epithelial sodium channel.
References
- 1.Oh Y, Warnock D G. J Am Soc Nephrol. 1997;8:126–129. doi: 10.1681/ASN.V81126. [DOI] [PubMed] [Google Scholar]
- 2.Ismailov I I, Berdiev B K, Fuller C M, Bradford A L, Lifton R P, Warnock D G, Bubien J K, Benos D J. Am J Physiol. 1996;270:C214–C223. doi: 10.1152/ajpcell.1996.270.1.C214. [DOI] [PubMed] [Google Scholar]
- 3.Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier B C. Nature (London) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 4.Schild L, Canessa C M, Shimkets R A, Gautschi I, Lifton R P, Rossier B C. Proc Natl Acad Sci USA. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimkets R A, Warnock D G, Bositis C M, Nelson-Williams C, Hansson J H, Schambelan M, Gill J R, Jr, Ulick S, Milora R V, Findling J W, Canessa C M, Rossier B C, Lifton R P. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 6.Hansson J H, Schild L, Lu Y, Wilson T A, Gautschi I, Shimkets R, Nelson-Williams C, Rossier B C, Lifton R P. Proc Natl Acad Sci USA. 1995;92:11495–11499. doi: 10.1073/pnas.92.25.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson J H, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton R P. Nat Genet. 1995;11:76–82. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]
- 8.Snyder P M, Price M P, McDonald F J, Adams C M, Volk K A, Zeiher B G, Stokes J B, Welsh M J. Cell. 1995;83:969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 9.Staub O, Dho S, Henry P C, Correa J, Ishikawa T, McGlade J, Rotin D. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 10.Schild L, Yu Y, Gautschi I, Schneeberger E, Lifton R P, Rossier B C. EMBO J. 1996;15:2381–2387. [PMC free article] [PubMed] [Google Scholar]
- 11.Schild L, Schneeberger E, Gautschi I, Firsov D. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Y R, Rutkowski M P, Klanke C A, Wu X M, Cui Y, Pun R Y K, Carter V, Reif M, Menon A G. J Am Soc Nephrol. 1996;7:2543–2549. doi: 10.1681/ASN.V7122543. [DOI] [PubMed] [Google Scholar]
- 13.McDonald F J, Price M P, Snyder P M, Welsh M J. Am J Physiol. 1995;268:C1157–C1163. doi: 10.1152/ajpcell.1995.268.5.C1157. [DOI] [PubMed] [Google Scholar]
- 14.Herbert J M, Augereau J M, Gleye J, Maffrand J P. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- 15.Bubien J K, Warnock D G. Am J Physiol. 1993;265:C1175–C1183. doi: 10.1152/ajpcell.1993.265.4.C1175. [DOI] [PubMed] [Google Scholar]
- 16.Fuller C M, Awayda M S, Arrate M P, Bradford A L, Morris R G, Canessa C M, Rossier B C, Benos D J. Am J Physiol. 1995;269:C641–C654. doi: 10.1152/ajpcell.1995.269.3.C641. [DOI] [PubMed] [Google Scholar]
- 17.Bubien J K, Jope R S, Warnock D G. J Biol Chem. 1994;269:17780–17783. [PubMed] [Google Scholar]
- 18.Awayda M S, Ismailov I I, Berdiev B K, Fuller C M, Benos D J. J Gen Physiol. 1996;108:49–65. doi: 10.1085/jgp.108.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bubien J K, Ismailov I I, Bakhram B K, Cornwell T, Lifton R P, Fuller C M, Achard J-M, Benos D J, Warnock D G. Am J Physiol. 1996;270:C208–C213. doi: 10.1152/ajpcell.1996.270.1.C208. [DOI] [PubMed] [Google Scholar]