Abstract
Three different stable lipoxin A4 (LXA4) analogs (i.e., 16-phenoxy-LXA4-Me, 15-cyclohexyl-LXA4-Me, and 15-R/S-methyl-LXA4-Me) were studied for their ability to modulate leukocyte-endothelial cell interactions in the rat mesenteric microvasculature. Superfusion of the rat mesentery with 50 μmol/liter NG-nitro-l-arginine methyl ester (l-NAME) caused a significant, time-dependent increase in leukocyte rolling (56 ± 8 cells/min; P < 0.01 vs. control) and leukocyte adherence (12.5 ± 1.2 cells/100 μm length of venule; P < 0.01 vs. control) after 120 min of superfusion. Concomitant superfusion of the rat mesentery with 10 nmol/liter of each of three lipoxin analogs consistently and markedly attenuated l-NAME-induced leukocyte rolling to 10 ± 4 (P < 0.01), 4 ± 1 (P < 0.01), and 32 ± 7 (P < 0.05) cells/min, and adherence to 4 ± 0.8 (P < 0.01), 1.1 ± 0.4 (P < 0.01), and 7 ± 0.7 (P < 0.05) cells/100 μm length of venule (16-phenoxy-LXA4-Me, 15-cyclohexyl-LXA4-Me, and 15-R/S- methyl-LXA4-Me, respectively). No alterations of systemic blood pressure or mesenteric venular shear rates were observed in any group. Immunohistochemical up-regulation of P-selectin expression on intestinal venular endothelium was significantly increased (P < 0.01) after exposure to l-NAME, and this was significantly attenuated by these lipoxin analogs (P < 0.01). Thus, in vivo superfusion of the rat mesentery with stable lipoxin analogs at 10 nmol/liter reduces l-NAME-induced leukocyte rolling and adherence in the mesenteric rat microvasculature by attenuating P-selectin expression. This anti-inflammatory mechanism may represent a novel and potent regulatory action of lipoxins on the immune system.
Keywords: intravital microscopy, nitric oxide synthase, adhesion molecules, immunohistochemistry
Lipoxins represent a relatively new class of arachidonic acid derivatives (i.e., trihydroxytetraene eicosanoids) that are produced by activated leukocytes, which, in turn, are able to modulate leukocyte functions (1). In vitro data indicate that one member of the naturally occurring lipoxins, (i.e., lipoxin A4, namely LXA4), inhibits both neutrophil and eosinophil chemotaxis at nanomolar concentrations (2, 3) and blocks polymorphonuclear neutrophil (PMN) transmigration across epithelial cells (4) and endothelial monolayers (5). Similar actions also are demonstrable in vivo where LXA4 exerts vasodilator properties (6–9), blocks both PMN diapedesis from postcapillary venules (10), and inhibits PMN entry in inflamed renal tissues in animal models of glomerulonephritis (11).
Nevertheless, we know of no specific information elucidating detailed mechanisms by which naturally occurring lipoxins and their synthetic stable analogs can directly modulate the interaction of leukocytes with vascular endothelial cells. LXA4, as with other autacoids, is rapidly inactivated in the local extracellular milieu. Analogs of LXA4 were designed and prepared by total organic synthesis so that they resist rapid inactivation and retain potent biological activities (12). A goal of this study was to use these metabolically stable analogs to investigate the in vivo mechanism of lipoxin action on leukocyte-endothelium interaction.
Leukocyte-endothelial cell interaction represents a multistep paradigm in which several adhesion glycoproteins (i.e., integrins, immunoglobulin superfamily members, and selectins) are involved. In particular, one member of the selectin family, P-selectin, is rapidly translocated from the Weibel–Palade bodies to the endothelial cell surface upon hypoxia-reoxygenation or activation with inflammatory mediators such as thrombin, histamine, or oxygen-derived free radicals (13–15). P-selectin promotes rolling of leukocytes, a key step in leukocyte-endothelium interaction, thus facilitating PMN adherence (13, 16). Leukocytes adhere to the endothelium, and some of them transmigrate, thus potentiating endothelial dysfunction and tissue injury (17, 18). Endothelial dysfunction, characterized by a reduced release of nitric oxide (NO), also has been shown to be critically related to increased leukocyte-endothelium interaction via up-regulation of endothelial cell adhesion molecules (19). We previously have established a functional relationship between the loss of endothelium-derived NO and the expression of P-selectin (20). Similarly, others have shown that blocking NO synthesis via NG-monomethyl-l-arginine or NG-nitro-l-arginine methyl ester (l-NAME) increases leukocyte adherence and emigration in the mesenteric microcirculation as well as enhances microvascular permeability (21, 22).
Therefore, the aim of the present study was to evaluate whether stable LXA4 analogs influence leukocyte-endothelial cell interaction and P-selectin expression in the rat mesenteric microvasculature and to ascertain the role of lipoxins on these two key early steps in leukocyte-endothelium interaction, namely leukocyte rolling and leukocyte adherence.
MATERIALS AND METHODS
Intravital Microscopy.
Male Sprague–Dawley rats, weighing 250–270 g, were anesthetized with sodium pentobarbital (40 mg/kg) injected intraperitoneally. A tracheotomy was performed to maintain a patent airway throughout the experiment. A polyethylene catheter was inserted in the left carotid artery to monitor mean arterial blood pressure. Mean arterial blood pressure was recorded on a Grass Model 7 oscillographic recorder using a Statham P23AC pressure transducer (Gould, Cleveland). The abdominal cavity was opened via a midline laparotomy, and a second incision was made through the skin and abdominal musculature on the right flank as described earlier (20).
A loop of ileal mesentery was exteriorized through the midline incision and placed in a temperature-controlled, fluid-filled Plexiglas chamber for observation of the mesenteric microcirculation via intravital microscopy. A jugular vein was cannulated for administration of supplemental sodium pentobarbital as needed to maintain a surgical plane of anesthesia throughout the experiment. The mesentery was placed over a Plexiglas pedestal in the superfusion chamber, and the ileum was secured with fine-gauge insect pins on a cork board for stabilization of the viewing field. The ileum and mesentery were superfused throughout the experiment with a modified Krebs–Henseleit solution (containing in mmol/liter: 118 NaCl, 4.74 KCl, 2.45 CaCl2, 1.19 KH2PO4, 1.19 MgSO4, and 12.5 NaHCO3) warmed to 37°C and bubbled with 95% N2 and 5% CO2.
A Nikon Microphot microscope was used to visualize the mesenteric microcirculation. The image was projected by a high-resolution video camera model XC77 (Hamamatsu, Hamamatsu, Japan) onto a Sony high-resolution video monitor, and the image recorded with a videocassette recorder. Red blood cell velocity was determined on-line using an optical Doppler velocimeter (23) obtained from the Microcirculation Research Institute, College Station, TX. This method gives an average red blood cell velocity, which is digitally displayed on a meter, and allows for the calculation of shear rates.
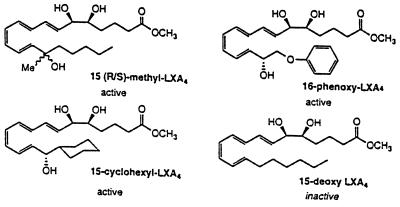
The rats were allowed to stabilize for 30 min after surgery. After stabilization, a 30–50 μm in diameter postcapillary venule was chosen for observation. A baseline recording was made to establish basal values for leukocyte rolling and adherence. The mesentery then was superfused with 50 μmol/liter l-NAME dissolved in Krebs–Henseleit solution for 120 min. Video recordings were made at 30, 60, 90, and 120 min after initiation of superfusion for quantification of leukocyte rolling and adherence. Rats were randomly divided into one of the following groups: (a) Krebs–Henseleit superfused rats, (b) 50 μmol/liter l-NAME superfused rats, (c) 50 μmol/liter l-NAME superfused rats also superfused with 10 nmol/liter 15-deoxy-LXA4-Me, an inactive lipoxin analog, and (d) 50 μmol/liter l-NAME superfused rats also superfused with either 1 and 10 nmol/liter of 16-phenoxy-LXA4-Me, 15-cyclohexyl-LXA4-Me, or 15-R/S-methyl-LXA4-Me. The LXA4 analogs were prepared by total organic synthesis, and their physical properties were confirmed by NMR and mass spectroscopy as described by Serhan et al. (12). The structural formulae for these four LXA4 analogs are shown in Fig. 1. Lipoxins are generated locally in vivo in humans and experimental animal models in picogram to nanogram quantities (1). Therefore, the concentrations used in the present studies were selected on the basis of in vitro actions of the LXA4 stable analogs and are in the range of the native lipoxins generated in vivo.
Figure 1.
Molecular structures of the four stable LXA4 analogs studied. The active lipoxin analogs are 15-cyclohexyl-LXA4-Me, 16-phenoxy-LXA4-Me, and 15-R/S-methyl-LXA4-Me. The inactive lipoxin analog is 15-deoxy-LXA4-Me.
The number of rolling and adhered leukocytes was determined off-line by playback of the videotape. Leukocytes were considered to be rolling if they were moving at a velocity significantly slower than that of red blood cells. Leukocyte rolling is expressed as the number of cells moving past a designated point per min (i.e., leukocyte flux). A leukocyte was judged to be adherent if it remained stationary for > 30 s. Adherence is expressed as the number of adherent leukocytes per 100 μm of vessel length. Red blood cell velocity (V) and venular diameter (D) were used to calculate venular wall shear rate (g), using the formula g = 8 (Vmean/D) where (Vmean = Vrbc/1.6), where V = velocity, and D = diameter (24).
Immunohistochemistry.
Immunohistochemical localization of P-selectin was determined after intravital microscopy was completed, using mAb PB1.3, which only detects surface expression of P-selectin. Both the superior mesenteric artery and superior mesenteric vein then were rapidly cannulated for perfusion fixation of the small bowel. The ileum was first washed free of blood by perfusion with Krebs–Henseleit buffer warmed to 37°C, bubbled with 95% O2 and 5% CO2, and fixed in 4% paraformaldehyde for 90 min at 4°C as previously described (25).
Immunohistochemical localization of P-selectin was accomplished using the avidin-biotin immunoperoxidase technique (Vectastain ABC Reagent, Vector Laboratories) as previously described by Weyrich et al. (25). Positive staining was defined as a venule displaying brown reaction product on greater than 50% of the circumference of its endothelium. Fifty venules per tissue section were examined, and 20 sections were analyzed per group, and the percentage of positive staining venules was tallied.
Data Analysis.
All data are presented as means ± SEM. Data were compared by ANOVA using posthoc analysis with Fisher’s corrected t test. All data on leukocyte rolling and adherence, arterial blood pressure, and shear rates were analyzed by ANOVA incorporating repeated measurements. Probabilities of 0.05 or less were considered statistically significant.
RESULTS
Intravital Microscopy.
Superfusion of the rat mesentery with 50 μmol/liter l-NAME over 120 min resulted in a time-dependent increase in leukocyte rolling in postcapillary venules of the rat mesenteric microvasculature (from 10 ± 4 cells/min to 56 ± 8 cells/min; P < 0.01). A similar time course also was observed for leukocyte adherence (from 2.2 ± 0.8 cells/100 μm to 12.5 ± 2 cells/100 μm; P < 0.01), clearly confirming that blockade of NO synthase in the rat mesenteric circulation resulted in a marked increase in leukocyte rolling and firm adherence of leukocytes to the vascular endothelium.
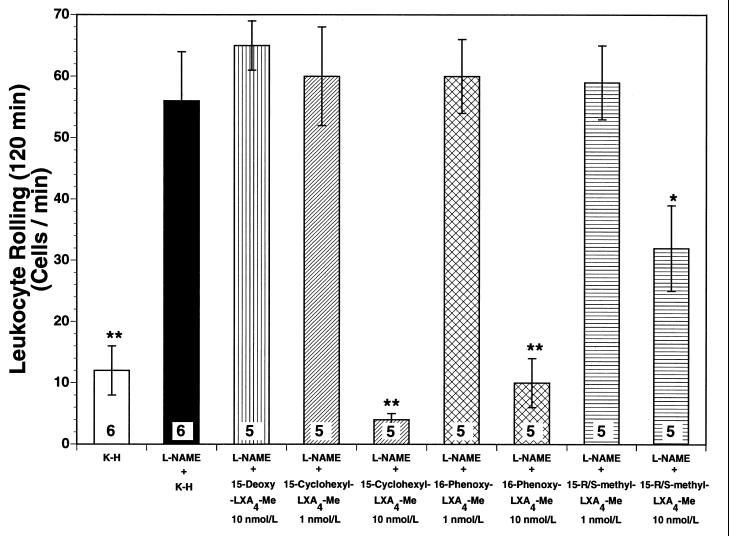
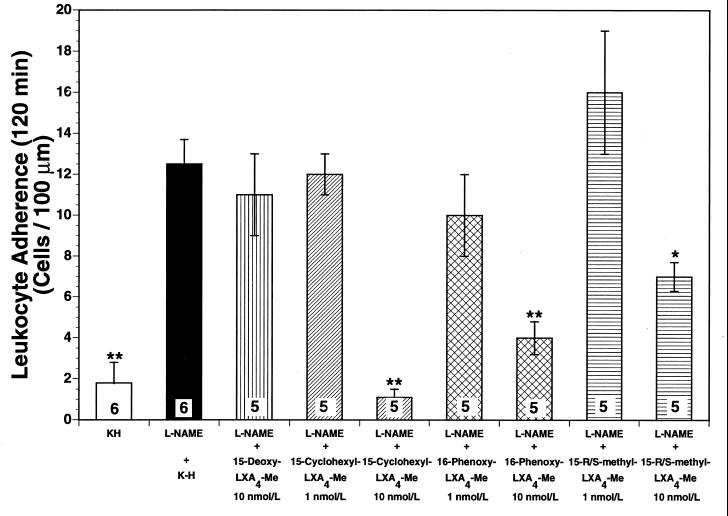
Superfusion of the rat mesentery with 10 nmol/liter 15-cyclohexyl-LXA4-Me, the most pharmacologically active lipoxin in our study, markedly attenuated the l-NAME-induced increases in leukocyte rolling (Fig. 2) by 93% (P < 0.01) and leukocyte adherence (Fig. 3) by 91% (P < 0.01), after 120 min of superfusion. However, when 15-cyclohexyl-LXA4-Me was applied to the mesenteric preparation at 1 nmol/liter it did not alter leukocyte rolling (Fig. 2) or leukocyte adherence (Fig. 3) during the 120-min observation period (not significant from l-NAME alone). This clearly indicates that the impact of lipoxins on leukocyte-endothelial cell interaction in the rat mesenteric microvasculature in vivo is likely to be concentration-dependent. Similar results were observed for the other two LXA4 stable analogs investigated. After 120 min of superfusion, both 16-phenoxy-LXA4-Me and 15-R/S-methyl-LXA4-Me inhibited leukocyte rolling (Fig. 2) by 82% (P < 0.01) and 39% (P < 0.05), respectively, and leukocyte adherence (Fig. 3) by 69% (P < 0.01) and 46% (P < 0.05), respectively.
Figure 2.
Leukocyte rolling after 120 min of superfusion. Superfusion of the rat mesentery with 50 μmol/liter l-NAME significantly increased leukocyte rolling in the rat mesenteric microvasculature [P > 0.01 vs. Krebs–Henseleit (K-H) solution-superfused control rats]. Concomitant superfusion of the rat mesentery with three different lipoxin analogs attenuated l-NAME-induced rolling in the following order of potency: 15-cyclohexyl-LXA4-Me>16-phenoxy-LXA4-Me>15-R/S-methyl-LXA4-Me. The control inactive lipoxin analog (15-deoxy-LXA4-Me) did not exert any significant effect on leukocyte rolling. Bar heights represent mean values; brackets indicate ± SEM. Numbers at base of bars indicate the numbers of rats studied in each group. ∗, P < 0.05 from l-NAME. ∗∗, P < 0.01 from l-NAME.
Figure 3.
Leukocyte adherence after 120-min superfusion. Superfusion of the rat mesentery with 50 μmol/liter l-NAME significantly increased leukocyte adherence in the rat mesenteric microvasculature [P > 0.01 vs. Krebs–Henseleit (K-H) solution-superfused control rats]. Concomitant superfusion of the rat mesentery with three different lipoxin analogs attenuated l-NAME-induced adherence in the following order of potency: 15-cyclohexyl-LXA4-Me>16-phenoxy-LXA4-Me>15-R/S-methyl-LXA4-Me. The control inactive lipoxin analog (15-deoxy-LXA4-Me) did not exert any significant effect on leukocyte rolling. Bar heights represent mean values; brackets indicate ± SEM. Numbers at base of bars indicate the numbers of rats studied in each group. ∗, P < 0.05 from l-NAME. ∗∗, P < 0.01 from l-NAME.
To exclude potential nonspecific actions of lipoxins on leukocyte-endothelium interaction in the rat mesenteric microcirculation, we also studied leukocyte rolling and adherence after superfusion of the rat mesentery with 15-deoxy-LXA4-Me, an inactive LXA4 structural analog that is chemically related to the active 15-cyclohexyl-LXA4-Me. Interestingly, at 10 nmol/liter, 15-deoxy-LXA4-Me was unable to attenuate l-NAME-induced leukocyte rolling (Fig. 2) and adherence (Fig. 3), thus confirming that the pharmacological properties observed for the three active lipoxin analogs were not due to nonspecific interaction of their molecular structures with biological systems.
There was no difference in the initial mean arterial blood pressure among the four groups of rats after all surgical procedures were performed. Mean arterial blood pressures ranged between 129 ± 7 and 134 ± 6 mmHg over the 2-hr observation period. Moreover, no significant systemic effect was observed after the exposure of the rat mesentery to either l-NAME alone or l-NAME plus any of the LXA4 analogs, as confirmed by the absence of any significant changes in mean arterial blood pressure over the 120-min observation period. Additionally, when venular shear rates for the different time-points were calculated in all experimental groups, no significant differences were recorded. Thus, venular diameters ranged from 39–50 μm in all groups, and venular shear rates varied between 575 to 625 (sec−1) in all groups. These findings clearly indicate that the adhesive interactions observed between leukocytes and endothelial cells were not due to changes in rheological factors, brought about by the superfusion of the rat mesentery with l-NAME, but most likely were due to enhanced expression of P-selectin on the surface of the venular endothelium.
Immunohistochemistry.
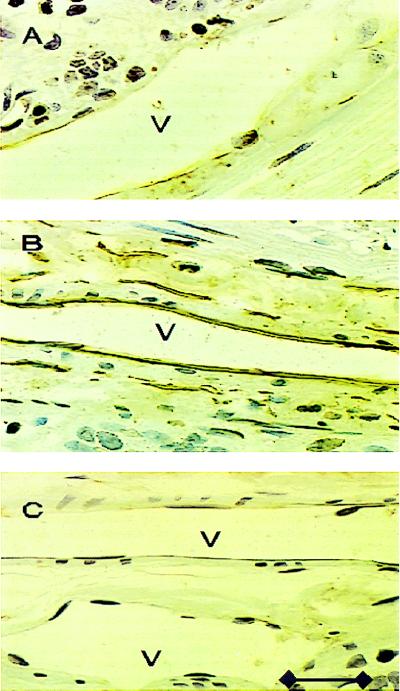
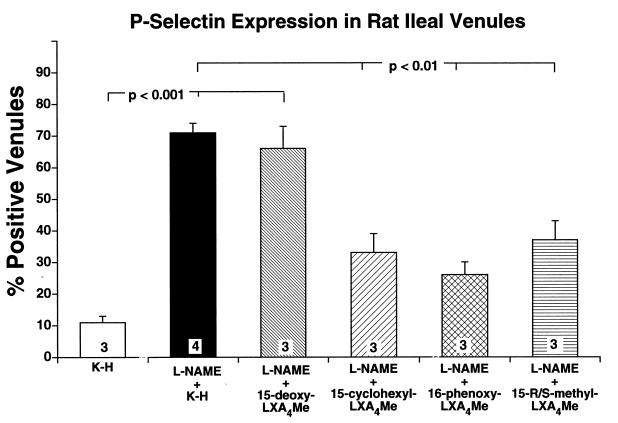
Expression of the endothelial cell adhesion molecule, P-selectin, was investigated on the ileal venular endothelium in control rats. Representative photomicrographs of venules from l-NAME superfused rats and l-NAME superfused rats given 16-phenoxy-LXA4-Me are shown in Fig. 4. Positive staining for this cell adhesion molecule was observed only on the venular endothelium in the rat ileum. The percentage of venules staining positively for P-selectin in ileal sections from control rats superfused only with Krebs–Henseleit buffer was consistently low (Fig. 5). However, superfusion with 50 μmol/liter l-NAME for 120 min resulted in an increased expression of P-selectin as quantified by the percentage of venules staining positively for P-selectin (P < 0.001). This represents a statistically significant increase in the surface expression of P-selectin under these experimental conditions (Fig. 5). This increased P-selectin expression on the venular endothelium was significantly attenuated by the simultaneous superfusion of 10 nmol/liter of any of the three stable LXA4 analogs, but not by the inactive LXA4 analog (Fig. 5). Thus, active LXA4 analogs significantly attenuated P-selectin surface expression in the rat mesenteric microvascular endothelium.
Figure 4.
Representative photomicrographs of rat ileal venules (V) showing immunolocalization of P-selectin expression. Control venule (A), l-NAME treated venule (B), and 16-phenoxy-LXA4-Me superfused l-NAME-treated venule (C). Brown reaction product indicates positive immunoperoxidase localization of P-selectin surface expression on the vascular endothelium (B). All three panels are lightly counterstained with Gill’s hematoxylin 2 and have the same magnification (×400). Calibration line in C represents 40 μm.
Figure 5.
Immunohistochemical summary of P-selectin expression on rat ileal venules. Percentage of venules staining positively for P-selectin in all experimental groups of rats. Bar heights represent mean values; brackets indicate ± SEM. Numbers at base of bars indicate the numbers of rats studied in each group. Twenty sections were studied in each rat.
DISCUSSION
We have described actions of three different pharmacologically active stable lipoxin analogs (i.e., 16-phenoxy-LXA4-Me, 15-cyclohexyl-LXA4-Me, and 15-R/S-methyl-LXA4-Me) on leukocyte-endothelial cell interactions induced by challenging the rat mesenteric microvasculature with the NO synthesis inhibitor l-NAME. These inhibitory actions on leukocyte rolling and adherence were found to be related to attenuation of the surface expression of the adhesion molecule P-selectin on the venular endothelial surface.
Recent studies on the leukocyte and endothelial adhesion molecules involved in the acute inflammatory response indicate a complex pattern of leukocyte-endothelium interaction that precedes emigration of leukocytes from the vasculature into the surrounding tissue. Leukocyte-endothelium interaction now is known to be a multistep process involving sequential activation of specific cell adhesion molecules (26, 27). P-selectin, a member of the selectin family of adhesion glycoproteins, is believed to play a significant role in the initial phase of leukocyte contact, which is rolling of leukocytes along the vascular endothelial surface (14, 20). PMN rolling serves to tether the unstimulated neutrophil to the activated endothelium, thus bringing the neutrophil in closer contact with the endothelial cells, allowing adherence to occur (13, 20).
Interestingly, a new class of lipid mediators (i.e., lipoxins) have been shown to be generated at sites of multicellular events by pathways that require cell-to-cell interactions (1). Under such conditions, these eicosanoids transmit transcellular bioregulatory signals, as demonstrated in in vitro models of platelet-neutrophil interaction (28). In contrast to other lipid mediators, which are proinflammatory substances, lipoxins have anti-inflammatory properties that may serve a counterregulatory role limiting the effects of inflammatory signals in neutrophil recruitment and tissue destruction (1, 11). Recently, the anti-inflammatory activity of lipoxins has been correlated to the expression of adhesion molecules. In this regard, 15-epi-lipoxins are potent inhibitors of leukotriene B4-mediated PMN adhesion to endothelial cells in vitro (12). Moreover, cell adhesion via selectins and integrins in conjunction with their respective cognate carbohydrate and immunoglobulin-like receptors strongly influences transcellular biosynthesis of lipoxins (1, 11). For example, lipoxin biosynthesis via platelet-PMN interactions during immunocomplex-induced glomerulonephritis is blocked by antibodies against P-selectin (11).
Therefore, we raised the question whether exogenously administered LXA4 stable analogs that resist rapid inactivation (12) could directly affect endothelial cell expression of adhesion molecules in vivo. In this connection, we activated endothelial cells in vivo by superfusing the rat mesentery with the NO synthesis inhibitor, l-NAME. Similar results have been obtained in rats subjected to ischemia-reperfusion of the mesentery. These rats were administered the same anti-P-selectin antibody intravenously as that used in the present study (29). In this regard, l-NAME has been effectively used to up-regulate leukocyte-endothelium interaction, because the increased adherence mediated by l-NAME is not due to direct leukocyte activation (19). Despite the fact that l-NAME inhibits NO synthase primarily in endothelial cells, up-regulation of P-selectin leads to enhanced leukocyte-endothelium interaction, which, in turn, leads to subsequent leukocyte activation (20).
In our study, LXA4-stable analogs significantly reduced l-NAME-provoked P-selectin expression in mesenteric endothelial cells and therefore attenuated leukocyte rolling and adherence along the mesenteric venular endothelium. These results agree with earlier in vitro observations, which demonstrated that lipoxins are potent inhibitors of PMN-endothelial cell interactions (5, 12) and also support more recent observations, which showed that LXA4-induced dilation of airway smooth muscle in vitro is partially exerted through NO generation (30). Moreover, our data help to explain many of the pharmacological activities attributed to lipoxins in experimental models of inflammation. PMN rolling promoted by P-selectin represents a key mechanism in initiating leukocyte-endothelial cell interactions and consequently leads to leukocyte activation. Activated leukocytes can release superoxide radicals, which can directly quench endogenous NO released by endothelial cells (31, 32), a process known to exacerbate endothelial dysfunction. Under these conditions, firm adherence of the neutrophils to the endothelial surface is initiated by expression of other adhesion molecules such as β2 integrins (i.e., CD11/CD18) on the PMN surface and intercellular adhesion molecule 1 on the endothelial cell surface (26, 33).
LXA4 analogs are designed to facilitate substitutions at carbon 15 through the ω 20 end of the native lipoxin structure. The lipoxins investigated in the present study are more resistant to cellular enzymatic conversion, and thus are pharmacologically more potent than naturally occurring lipoxins (12, 34, 35). Results from in vitro studies also have suggested that nanomolar concentrations of synthetic LXA4 analogs are able to inhibit PMN adhesion and subsequent transmigration (5, 12, 34). In this regard, our study demonstrates the high pharmacologic potency of these molecules in vivo, (e.g., the inhibition of l-NAME-induced leukocyte rolling and adherence). Taken together, these data clearly support the concept that lipoxins are potent inhibitors of surface expression of P-selectin on endothelial cells. This may be a key mechanism by which lipoxins inhibit leukocyte-endothelial interaction in inflammatory disease states. However, another possibility may exist in which lipoxins may also reduce leukocyte reactivity directly.
In conclusion, this study demonstrates that in vivo administration of stable lipoxin analogs inhibits leukocyte rolling and adherence in the rat mesenteric circulation. These effects were found to be mediated at low nanomolar concentrations via down-regulation of P-selectin present in endothelial cells. Stable lipoxin analogs may represent a new class of potent pharmacological agents useful for studying leukocyte-endothelial cell interaction and also may represent a novel strategy for modulating the pathophysiology of leukocyte-induced endothelial dysfunction in inflammatory and circulatory disorders.
Acknowledgments
We thank Valery Fokin for skillful contributions to the total synthesis of the lipoxin analogs. This work was supported by Research Grant No. GM45434 (A.M.L.) and GM38765 (C.N.S.) from the National Institute of General Medical Science of the National Institutes of Health. C.N.S. and N.A.P. also acknowledge a discovery research grant from Schering–Berlex.
ABBREVIATIONS
- l-NAME
NG-nitro-l-arginine methyl ester
- LXA4
lipoxin A4
- PMN
polymorphonuclear neutrophil
References
- 1.Serhan C N. Biochim Biophys Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 2.Lee T H, Horton C E, Kyan-Aung C E, Haskard D, Crea A E G, Spur B W. Clin Sci. 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 3.Soyombo O, Spur B W, Lee T H. Allergy. 1994;49:230–234. doi: 10.1111/j.1398-9995.1994.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 4.Colgan S P, Serhan C N, Parkos C A, Delp-Archer C, Madara J L. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papayianni A, Serhan C N, Brady H R. J Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- 6.Badr K F, Lakkis F G. Ann NY Acad Sci. 1994;744:216–228. doi: 10.1111/j.1749-6632.1994.tb52739.x. [DOI] [PubMed] [Google Scholar]
- 7.Dahlén S E. Adv Prostaglandin Thromboxane Leukotriene Res. 1989;19:122–127. [PubMed] [Google Scholar]
- 8.Hedqvist P, Raud J, Palmertz U, Haeggström J, Nicolaou K, Dahlén S E. Acta Physiol Scand. 1989;137:571–572. doi: 10.1111/j.1748-1716.1989.tb08805.x. [DOI] [PubMed] [Google Scholar]
- 9.Lefer A M, Stahl G L, Lefer D J, Brezinski M E, Nicolaou K C, Veale C A, Abe Y, Smith J B. Proc Natl Acad Sci USA. 1988;85:8340–8344. doi: 10.1073/pnas.85.21.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raud J, Palmertz U, Dahlén S E, Hedqvist P. Adv Exp Med Biol. 1991;314:185–192. doi: 10.1007/978-1-4684-6024-7_11. [DOI] [PubMed] [Google Scholar]
- 11.Papayianni A, Serhan C N, Phillips M L, Rennke H G, Brady H R. Kidney Int. 1995;47:1295–1302. doi: 10.1038/ki.1995.184. [DOI] [PubMed] [Google Scholar]
- 12.Serhan C N, Maddox J F, Petasis N A, Akritopoulou-Zanze I, Papayianni A, Brady H R, Colgan S P, Madara J L. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 13.Lorant D E, Patel K D, McIntyre T M, McEver R P, Prescott S M, Zimmerman G A. J Cell Biol. 1991;115:223–228. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEver R P, Beckstead J H, Moore K L, Marshall-Carson L, Bainton D F. J Clin Invest. 1989;84:92–97. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel K D, Zimmerman G A, Prescott S M, McEver R P, McIntyre T M. J Cell Biol. 1991;112:749–754. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorant D E, Topham M K, Whatley R E, McEver R P, McIntyre T M, Prescott S M, Zimmerman G A. J Clin Invest. 1993;92:559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Entman M L, Michael L, Rossen R D, Dreyer W J, Anderson D C, Smith C W. FASEB J. 1991;5:2529–2534. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- 18.Aoki N, Johnson G, III, Lefer A M. Am J Physiol. 1990;258:G275–G284. doi: 10.1152/ajpgi.1990.258.2.G275. [DOI] [PubMed] [Google Scholar]
- 19.Kubes P, Suzuki M, Granger D N. Proc Natl Acad Sci USA. 1991;88:4651–4656. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davenpeck K L, Gauthier T W, Lefer A M. Gastroenterology. 1994;107:1050–1058. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 21.Kubes P, Granger D N. Am J Physiol. 1992;262:H611–H618. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 22.Kurose I, Kubes P, Wolf R, Anderson D C, Paulson M, Miyasaka M, Granger D N. Circ Res. 1993;73:164–169. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- 23.Borders J L, Granger H J. Microvasc Res. 1984;27:117–222. doi: 10.1016/0026-2862(84)90047-5. [DOI] [PubMed] [Google Scholar]
- 24.Granger D N, Benoit J N, Suzuki M, Grisham M B. Am J Physiol. 1989;257:G683–G689. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- 25.Weyrich A S, Ma X, Lefer D J, Albertine K H, Lefer A M. J Clin Invest. 1993;91:2620–2628. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butcher E C. In: Mechanism of Lymphocyte Activation and Immune Regulation IV: Cellular Communications. Gupta S, Waldmann T A, editors. New York: Plenum; 1992. pp. 181–194. [Google Scholar]
- 27.Von Adrian, U. H., Chambers, J. D., McEvory, L. M., Bargatze, R. F., Arfors, K. & Butcher, E. C. (1991) Proc. Natl. Acad. Sci. USA 7538–7542. [DOI] [PMC free article] [PubMed]
- 28.Marcus A J, Serhan C N. Biochem Biophys Res Commun. 1982;109:130–137. doi: 10.1016/0006-291x(82)91575-3. [DOI] [PubMed] [Google Scholar]
- 29.Davenpeck K L, Gauthier T W, Albertine K H, Lefer A M. Am J Physiol. 1994;267:H622–H630. doi: 10.1152/ajpheart.1994.267.2.H622. [DOI] [PubMed] [Google Scholar]
- 30.Tamaoki J, Tagaya E, Yamawaki I, Konno K. Eur J Pharmacol. 1995;287:233–238. doi: 10.1016/0014-2999(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 31.Rubanyi G M, Vanhoutte P M. Am J Physiol. 1987;250:H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, Weyrich A S, Lefer D J, Lefer A M. Circ Res. 1993;72:403–409. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence M B, Springer T A. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 34.Fiore S, Serhan C N. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 35.Serhan C N, Haeggström J Z, Leslie C C. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]