Abstract
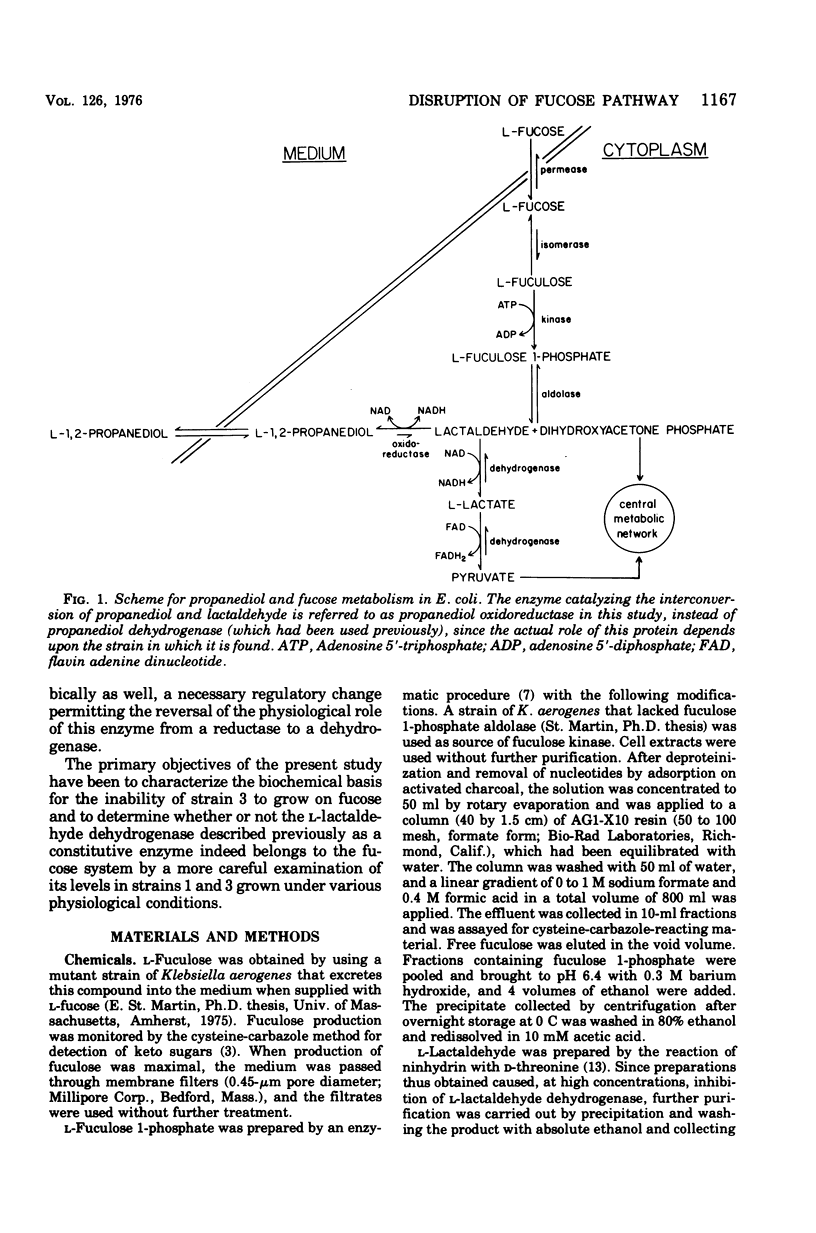
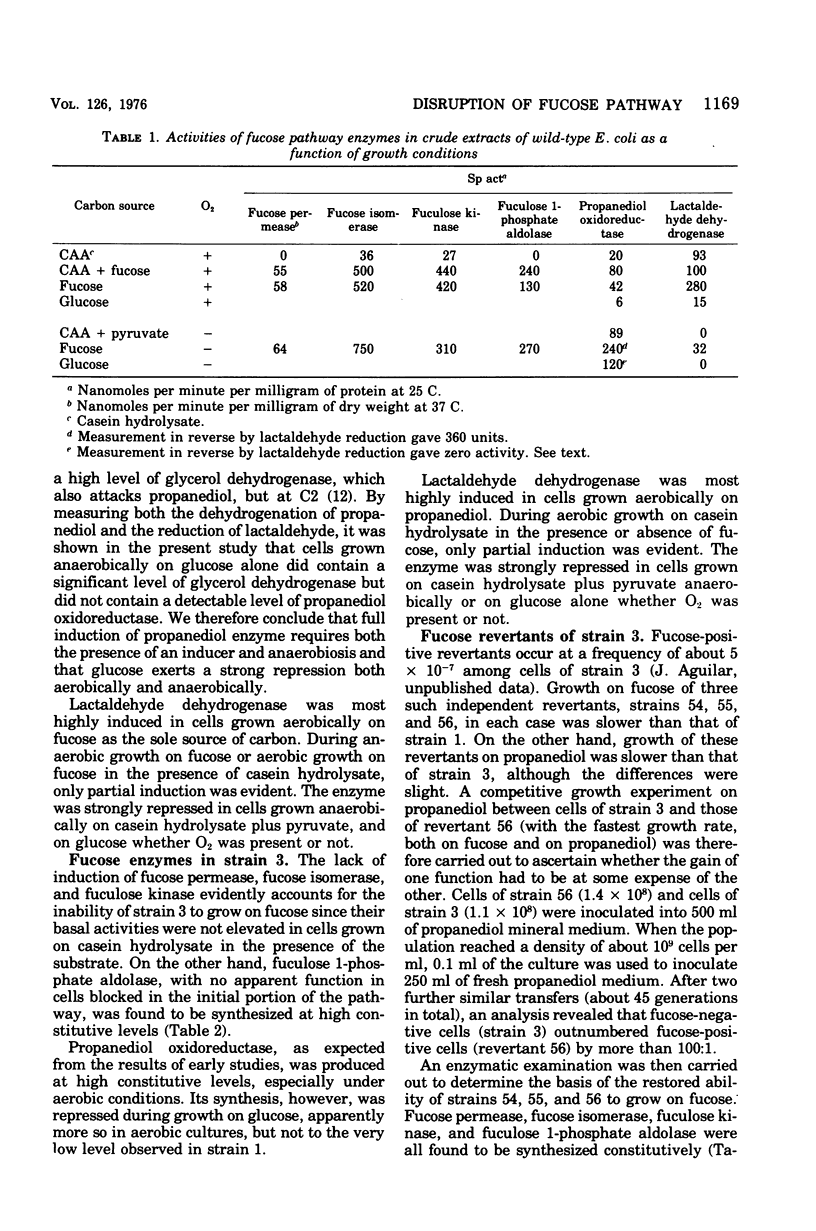
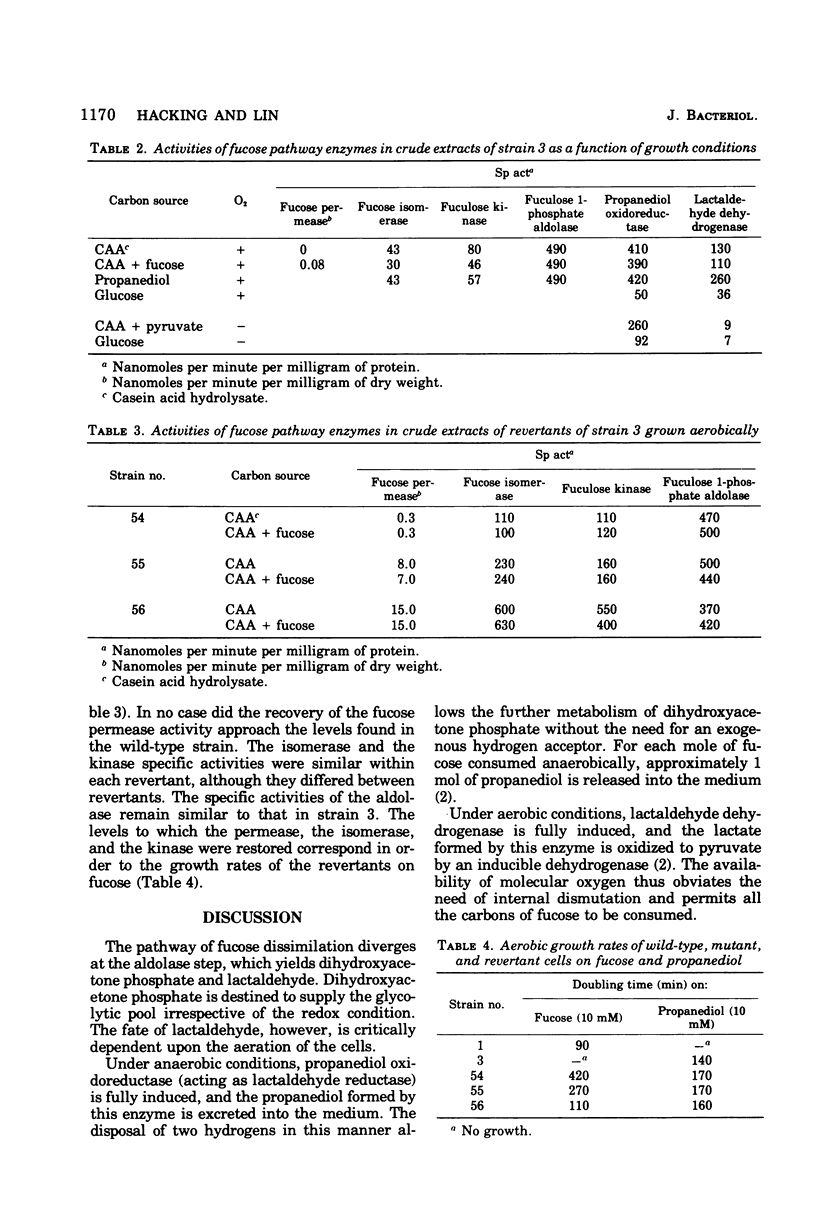
In Escherichia coli, L-fucose is dissimilated via an inducible pathway mediated by L-fucose permease, L-fucose isomerase, L-fucose kinase, and L-fuculose 1-phosphate aldolase. The last enzyme cleaves the six-carbon substrate into dihydroxyacetone phosphate and L-lactaldehyde. Aerobically, lactaldehyde is oxidized to L-lactate by a nicotinamide adenine dinucleotide (NAD)-linked dehydrogenase. Anaerobically, lactaldehyde is reduced by an NADH-COUPLED REDUCTASE TO L-1,2-propanediol, which is lost into the medium irretrievably, even when oxygen is subsequently introduced. Propanediol excretion is thus the end result of a dismutation that permits further anaerobic metabolism of dihydroxy-acetone phosphate. A mutant selected for its ability to grow aerobically on propanediol as a carbon and energy source was reported to produce lactaldehyde reductase constitutively and at high levels, even aerobically. Under the new situation, this enzyme serves as a propanediol dehydrogenase. It was also reported that the mutant had lost the ability to grow on fucose. In the present study, it is shown that in wild-type cells the full synthesis of lactaldehyde dehydrogenase requires the presence of both molecular oxygen and a small molecule effector, and the full synthesis of lactaldehyde reductase requires anaerobiosis and the presence of a small molecule effector. The failure of mutant cells to grow on fucose reflects the impairment of a regulatory element in the fucose system that prevents the induction of the permease, the isomerase, and the kinase. The aldolase, on the other hand, is constitutively synthesized. Three independent fucose-utilizing revertants of the mutant all produce the permease, the isomerase, the kinase, as well as the aldolase, constitutively. These strains grow less well than the parental mutant on propanediol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cocks G. T., Aguilar T., Lin E. C. Evolution of L-1, 2-propanediol catabolism in Escherichia coli by recruitment of enzymes for L-fucose and L-lactate metabolism. J Bacteriol. 1974 Apr;118(1):83–88. doi: 10.1128/jb.118.1.83-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- HEATH E. C., GHALAMBOR M. A. The metabolism of L-fucose. I. The purification and properties of L-fuculose kinase. J Biol Chem. 1962 Aug;237:2423–2426. [PubMed] [Google Scholar]
- HUFF E. The metabolism of 1,2-propanediol. Biochim Biophys Acta. 1961 Apr 15;48:506–517. doi: 10.1016/0006-3002(61)90048-8. [DOI] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T. Purification and properties of lactaldehyde dehydrogenase from Escherichia coli. J Biol Chem. 1969 Oct 10;244(19):5233–5238. [PubMed] [Google Scholar]
- Zagalak B., Frey P. A., Karabatsos G. L., Abeles R. H. The stereochemistry of the conversion of D and L 1,2-propanediols to propionaldehyde. J Biol Chem. 1966 Jul 10;241(13):3028–3035. [PubMed] [Google Scholar]



