Abstract
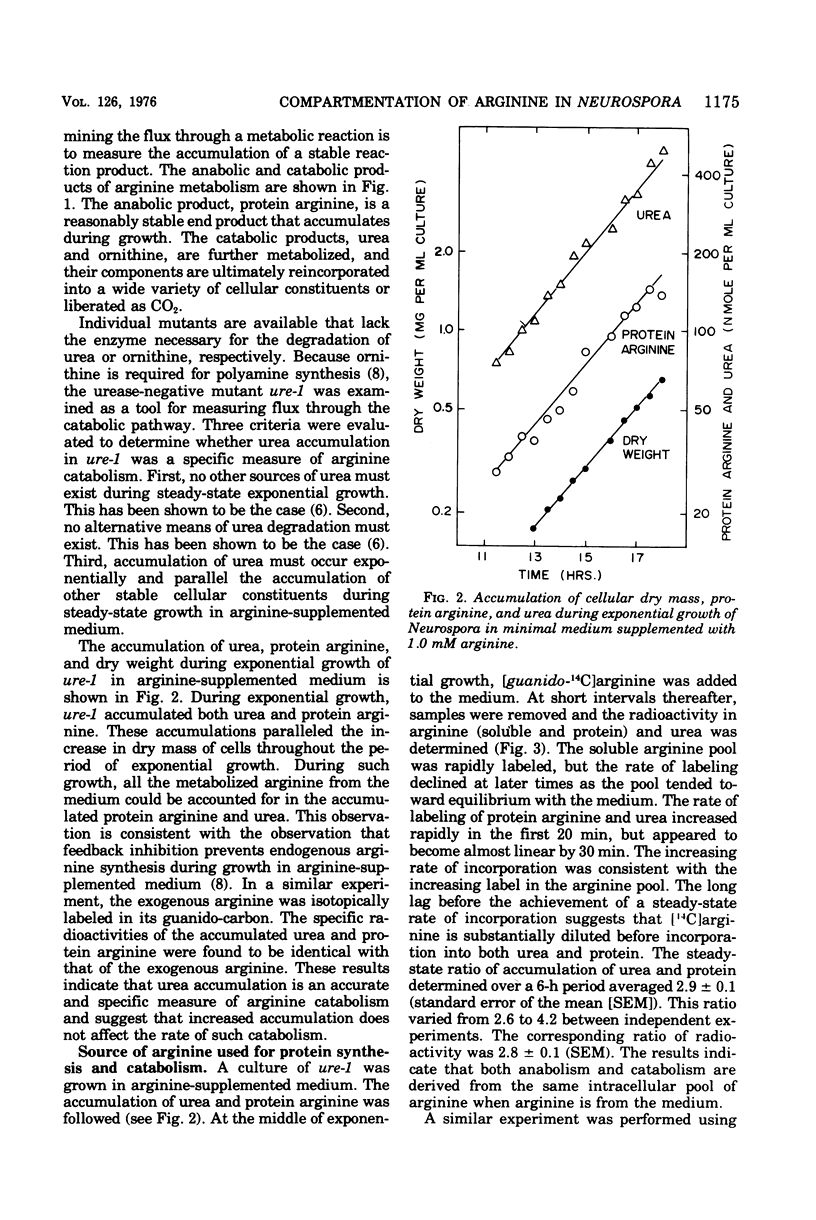
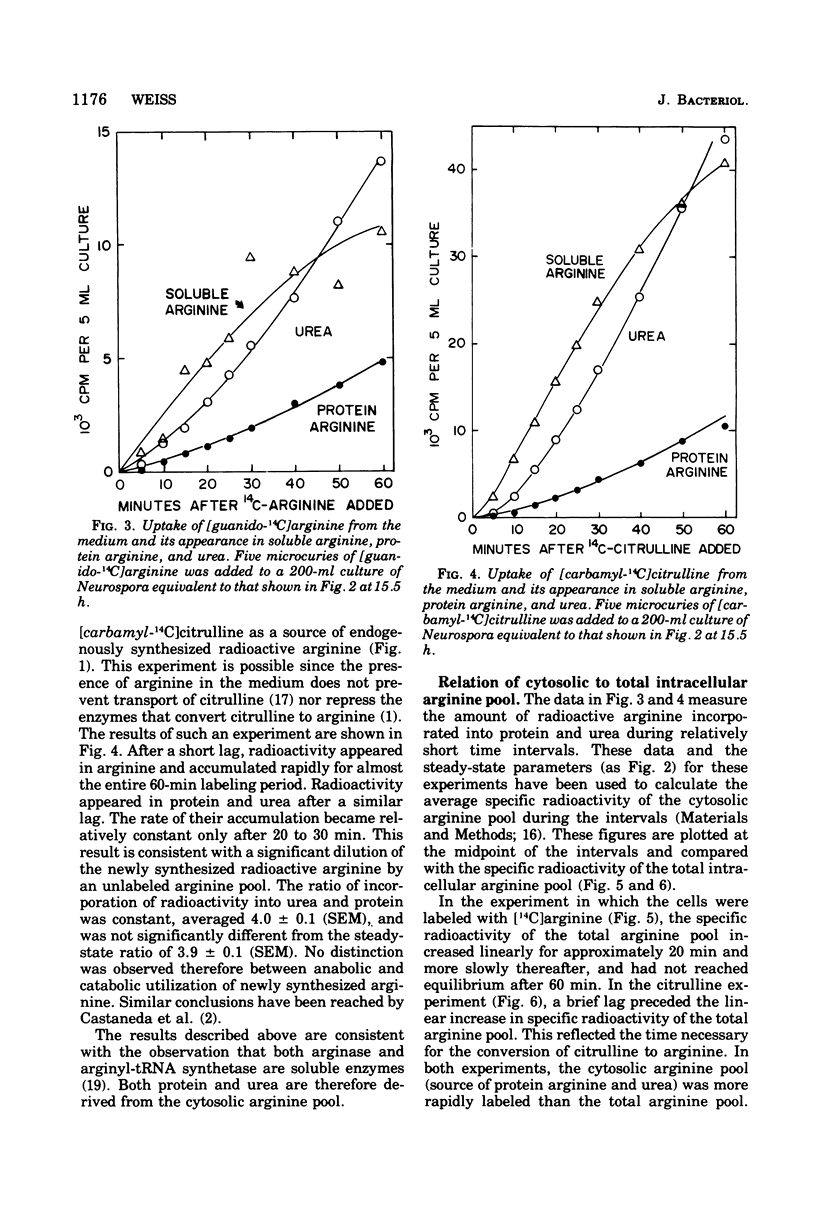
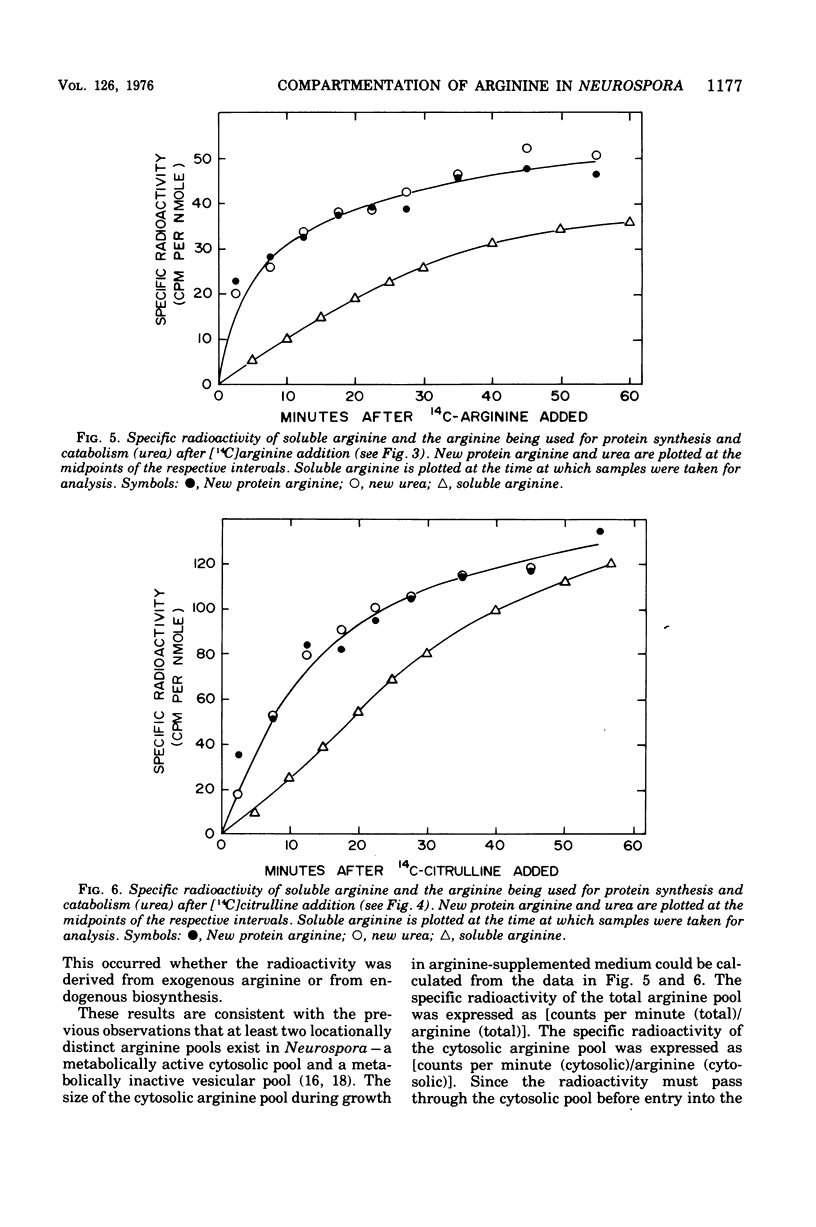
The fate of [14-C]arginine derived from the medium or from biosynthesis has been examined in Neurospora growing in arginine-supplemented medium. In both cases the label enters the cytosol, where it is used efficiently for both protein synthesis and catabolism before mixing with the majority of the endogenous [12C]arginine pool. Both metabolic processes appear to use the same cytosolic arginine pool. It is calculated that the nonorganellar cytoplasm contains approximately 20% of the intracellular arginine pool when the cells are growing in arginine-supplemented medium. The results suggest that compartmentation of arginine is a significant factor in controlling arginine metabolism in Neurospora. The significance of these results for studies of amino acid metabolism in other eukaryotic systems is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthelmess I. B., Curtis C. F., Kacser H. Control of the flux to arginine in Neurospora crassa: de-repression of the last three enzymes of the arginine pathway. J Mol Biol. 1974 Aug 5;87(2):303–316. doi: 10.1016/0022-2836(74)90151-x. [DOI] [PubMed] [Google Scholar]
- CROKAERT R., SCHRAM E. Dosage des N-carbamoyldérivés d'acides aminés par la diacétylmonoxime. Bull Soc Chim Biol (Paris) 1958;40(7-8):1093–1106. [PubMed] [Google Scholar]
- Castañeda M., Martuscelli J., Mora J. The catabolism of L-arginine by Neurospora crassa. Biochim Biophys Acta. 1967 Jul 25;141(2):276–286. doi: 10.1016/0304-4165(67)90102-x. [DOI] [PubMed] [Google Scholar]
- Cybis J. J., Davis R. H. Acetylglutamate kinase: a feedback-sensitive enzyme of arginine biosynthesis in Neurospora. Biochem Biophys Res Commun. 1974 Sep 23;60(2):629–634. doi: 10.1016/0006-291x(74)90287-3. [DOI] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Sources of urea in Neurospora. Biochim Biophys Acta. 1970 Aug 14;215(2):412–414. doi: 10.1016/0304-4165(70)90042-5. [DOI] [PubMed] [Google Scholar]
- HENDLER R. W. A model for protein synthesis. Nature. 1962 Mar 3;193:821–823. doi: 10.1038/193821a0. [DOI] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- KORITZ S. B., COHEN P. P. Colorimetric determination of carbamylamino acids and related compounds. J Biol Chem. 1954 Jul;209(1):145–150. [PubMed] [Google Scholar]
- Kolmark H. G. Genetic studies of urease mutants in Neurospora crassa. Mutat Res. 1969 Jul-Aug;8(1):51–63. doi: 10.1016/0027-5107(69)90140-7. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Woodside K. H., Henry J. E. Compartmentation of free valine and its relation to protein turnover in perfused rat liver. J Biol Chem. 1972 May 10;247(9):2776–2784. [PubMed] [Google Scholar]
- SLAYMAN C. W., TATUM E. L. POTASSIUM TRANSPORT IN NEUROSPORA. I. INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS, AND CATION REQUIREMENTS FOR GROWTH. Biochim Biophys Acta. 1964 Nov 29;88:578–592. [PubMed] [Google Scholar]
- Srere P. A., Mosbach K. Metabolic compartmentation: symbiotic, organellar, multienzymic, and microenvironmental. Annu Rev Microbiol. 1974;28(0):61–83. doi: 10.1146/annurev.mi.28.100174.000425. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites W. M., Pendyala L. Regulation of amino acid assimilation in a strain of Neurospora crassa lacking basic amino acid transport activity. Biochim Biophys Acta. 1969 Dec 30;192(3):455–461. doi: 10.1016/0304-4165(69)90394-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]


