Abstract
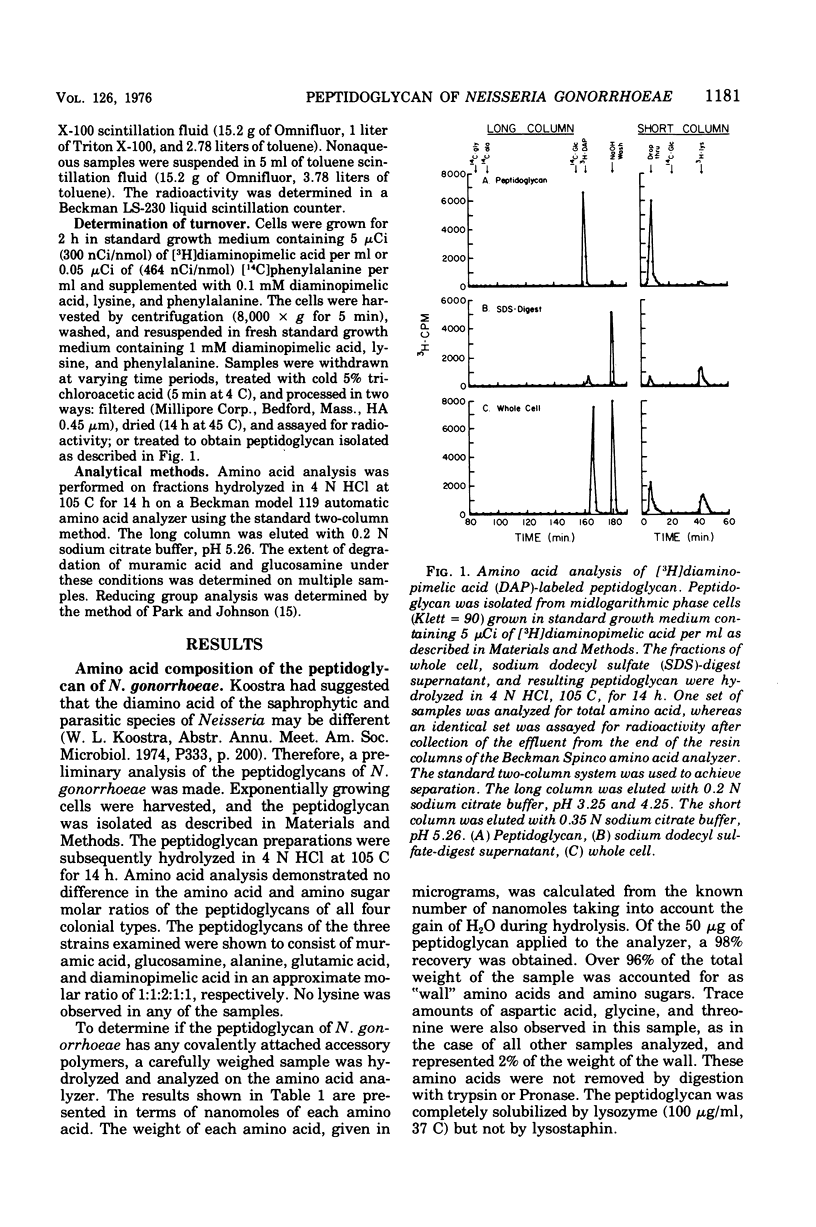
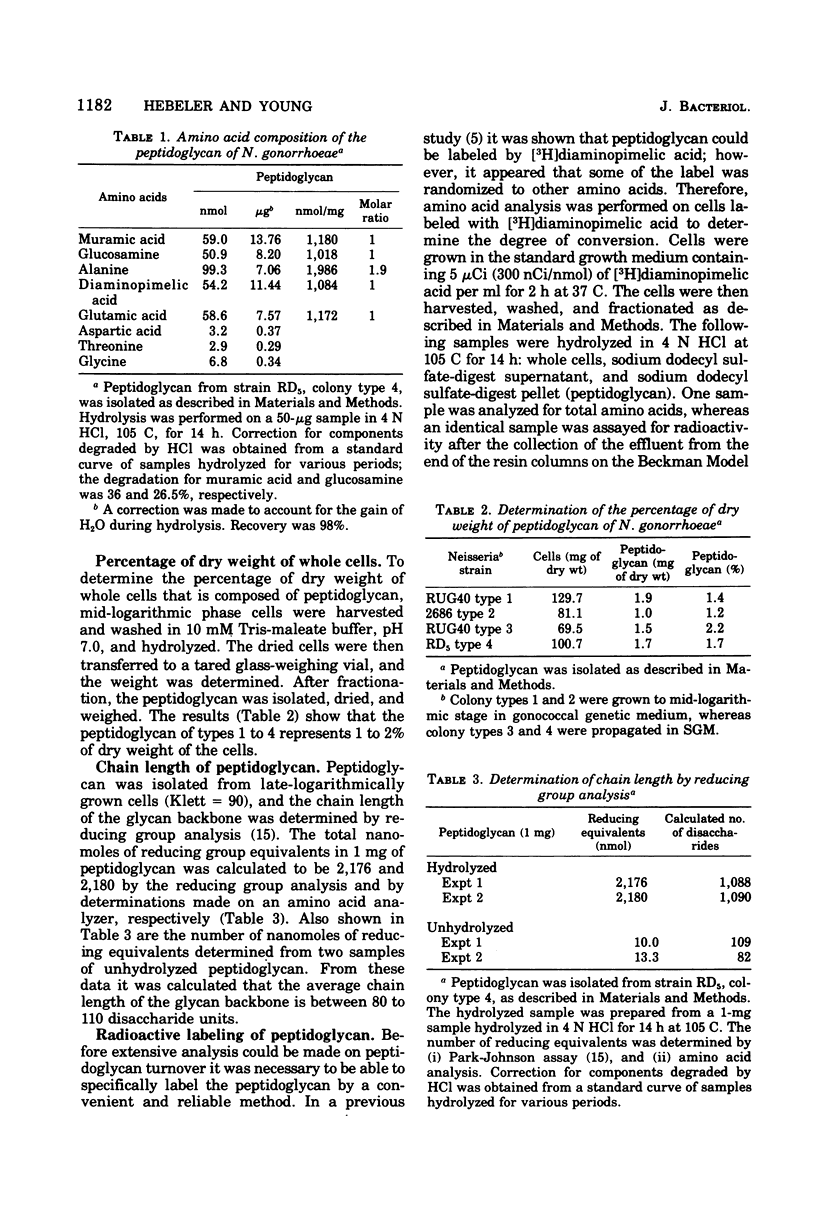
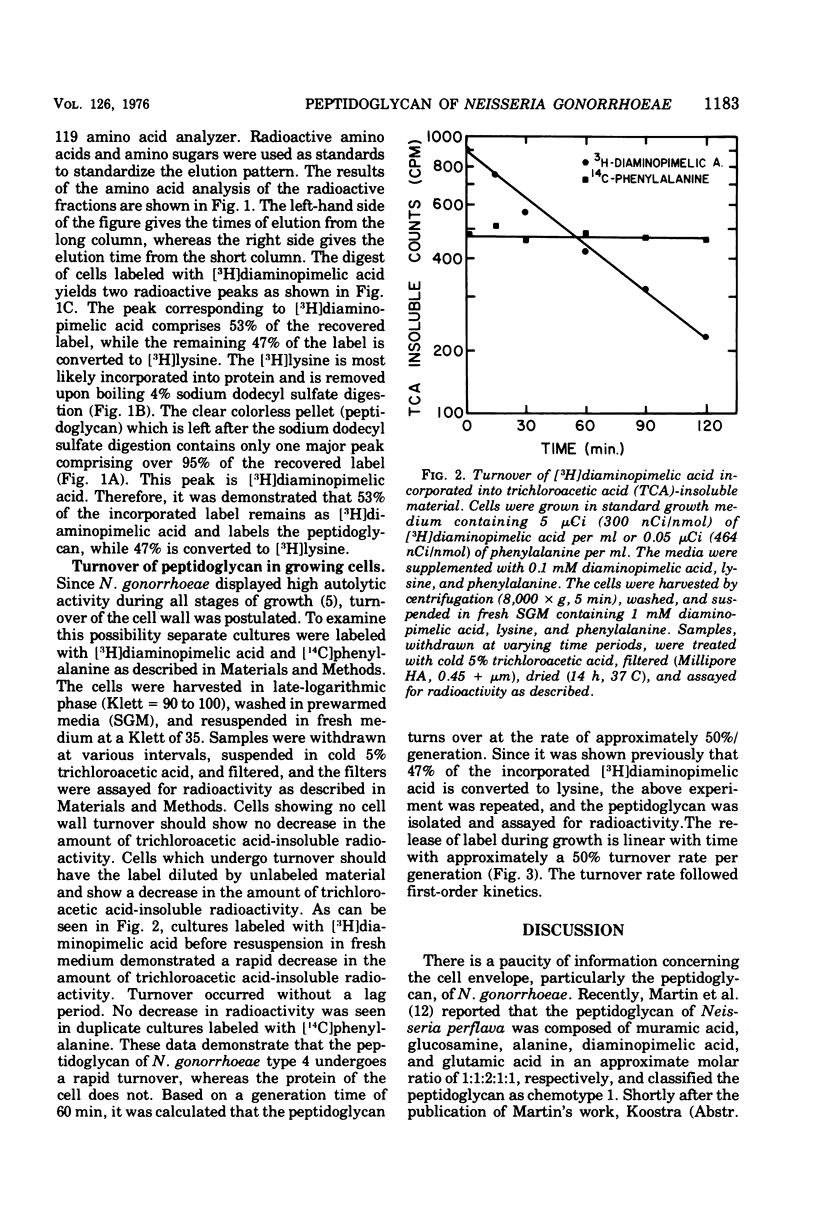
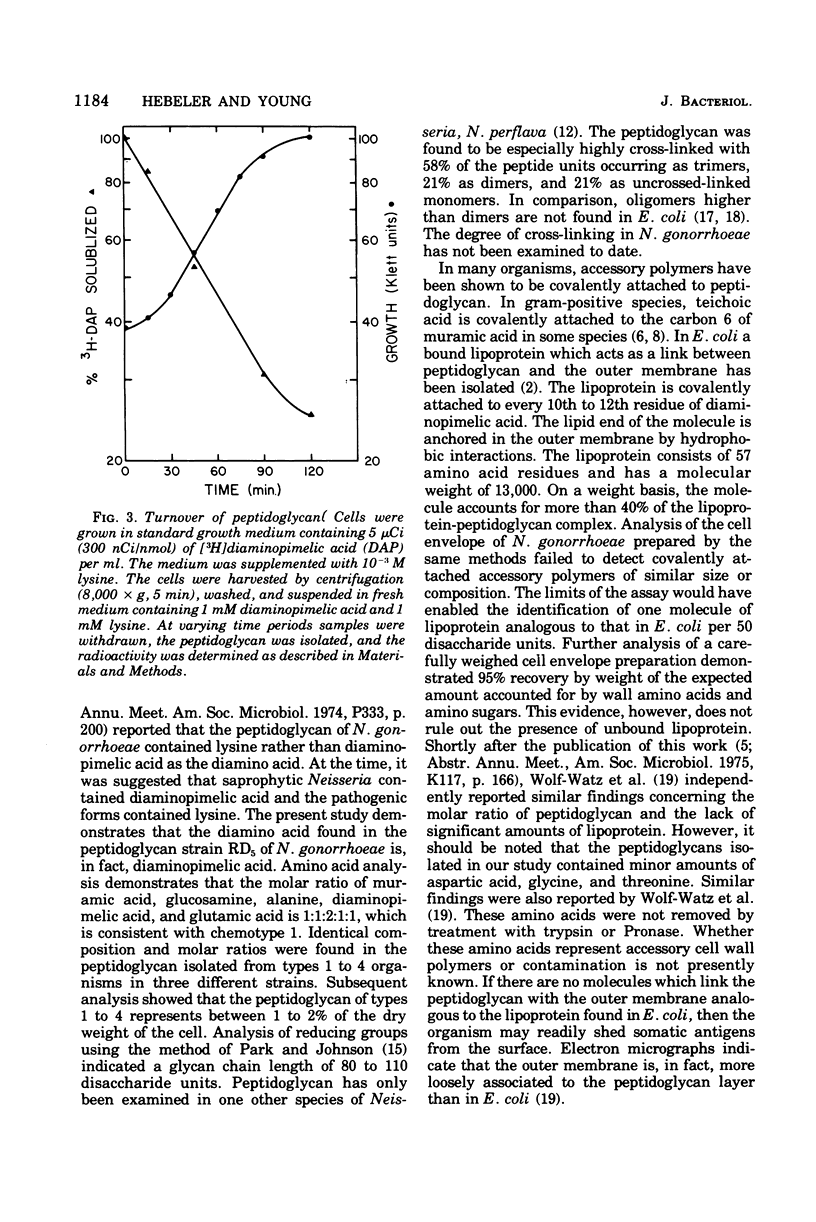
The peptidoglycan of all four colonial types of a number of strains of Neisseria gonorrhoeae constituted 1 to 2% of the dry weight of the cell. The chemical composition of cell types examined was similar with molar ratios of 1:1:2:1:1 for muramic acid, glucosamine, alanine, glutamic acid, and diaminopimelic acid, respectively. Ninety-six percent of the mass of the peptidoglycan was composed of these compounds. A lipoprotein analogous to that observed in Escherichia coli was not detected. The chain length of the glycan varied from 80 to 110 disaccharide units. The peptide contained equimolar amounts of D- and L-alanine. The rate of turnover of peptidoglycan in strain RD5 was 50% per generation. Turnover proceeded without a lag and followed first-order kinetics.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Braun V., Bosch V. Sequence of the murein-lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972 Jun 23;28(1):51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Turnover of mucopeptide during the life cycle of Bacillus megaterium. Folia Microbiol (Praha) 1971;16(5):372–382. doi: 10.1007/BF02875757. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Strnadová M. Turnover of murein in a diaminopimelic acid dependent mutant of Escherichia coli. Folia Microbiol (Praha) 1972;17(6):446–455. doi: 10.1007/BF02872729. [DOI] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975 May;122(2):385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Linkage between the teichuronic acid and mucopeptide components. Biochem J. 1970 Apr;117(3):431–439. doi: 10.1042/bj1170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hall E. A. The linkage between the polysaccharide and mucopeptide components of the cell wall of Lactobacillus casei. Biochem J. 1965 Aug;96(2):302–309. doi: 10.1042/bj0960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Dul M. J., Young F. E. Stability of pathogenic colony types of Neisseria gonorrhoeae in liquid culture by using the parameters of colonial morphology and deoxyribonucleic acid transformation. J Clin Microbiol. 1975 Feb;1(2):165–170. doi: 10.1128/jcm.1.2.165-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. P., Fleck J., Mock M., Ghuysen J. M. The wall peptidoglycans of Neisseria perflava, Moraxella glucidolytica, Pseudomonas alcaligenes and Proteus vulgaris strain P18. Eur J Biochem. 1973 Oct 5;38(2):301–306. doi: 10.1111/j.1432-1033.1973.tb03062.x. [DOI] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Glaser L. Turnover of the cell wall of Bacillus subtilis W-23 during logarithmic growth. Biochem Biophys Res Commun. 1970 May 22;39(4):699–706. doi: 10.1016/0006-291x(70)90261-5. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Van Heijenoort J., Elbaz L., Dezélée P., Petit J. F., Bricas E., Ghuysen J. M. Structure of the meso-diaminopimelic acid containing peptidoglycans in Escherichia coli B and Bacillus megaterium KM. Biochemistry. 1969 Jan;8(1):207–213. doi: 10.1021/bi00829a030. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



