Abstract
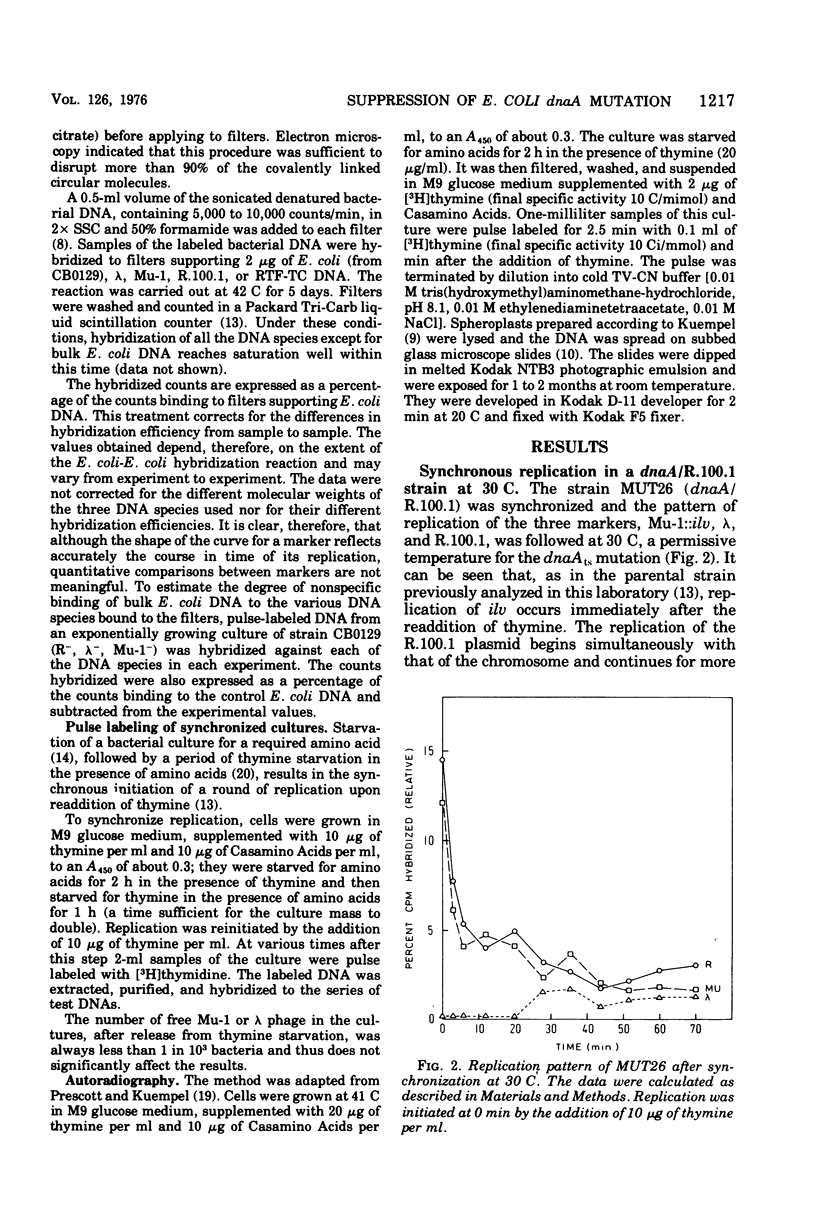
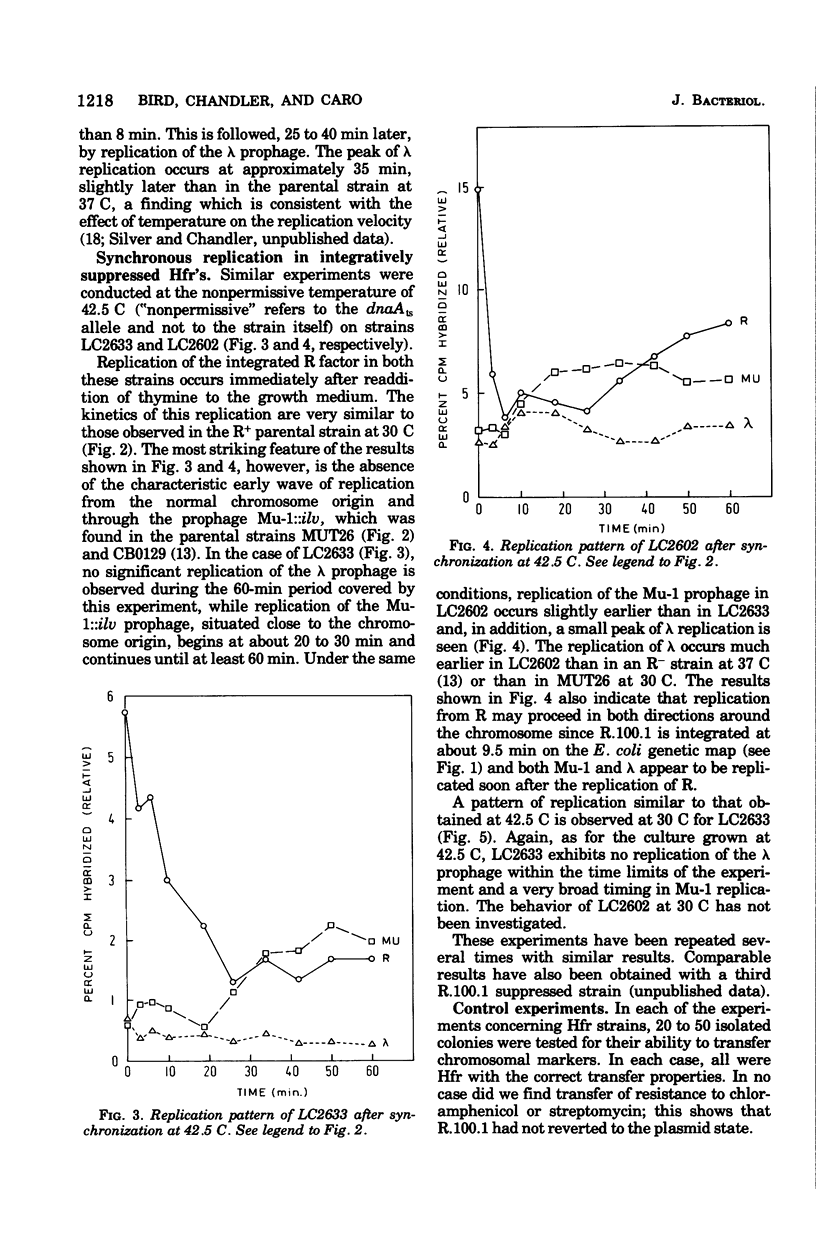
We have followed, by deoxyribonucleic acid-deoxyribonucleic acid hybridization, the order of replication of three chromosomal markers during a synchronous round of replication in three strains of Escherichia coli carrying a dnaAts mutation: one strain in which the F-like R factor R.100.1 was established as a plasmid and two strains in which the dnaA mutation was suppressed by the integration of R.100.1 into the chromosome. In the R+ strain at 30C, replication of the plasmid took place simultaneously with the initiation of chromosome replication at the normal origin. In the integratively suppressed Hfr strains, at 42.5 C, chromosome replication was initiated preferentially from the integrated plasmid; little or no initiation occurred at the normal origin. Similar results were obtained for the one strain tested at 30 C. For both Hfr strains at 42.5 C, the data suggest that at least part of the population replicated bidirectionally. This conclusion had been confirmed using an autoradiographic procedure. Both types of experiment indicate a wide variation in the rate of travel of individual replication forks within the population.
Full text
PDF
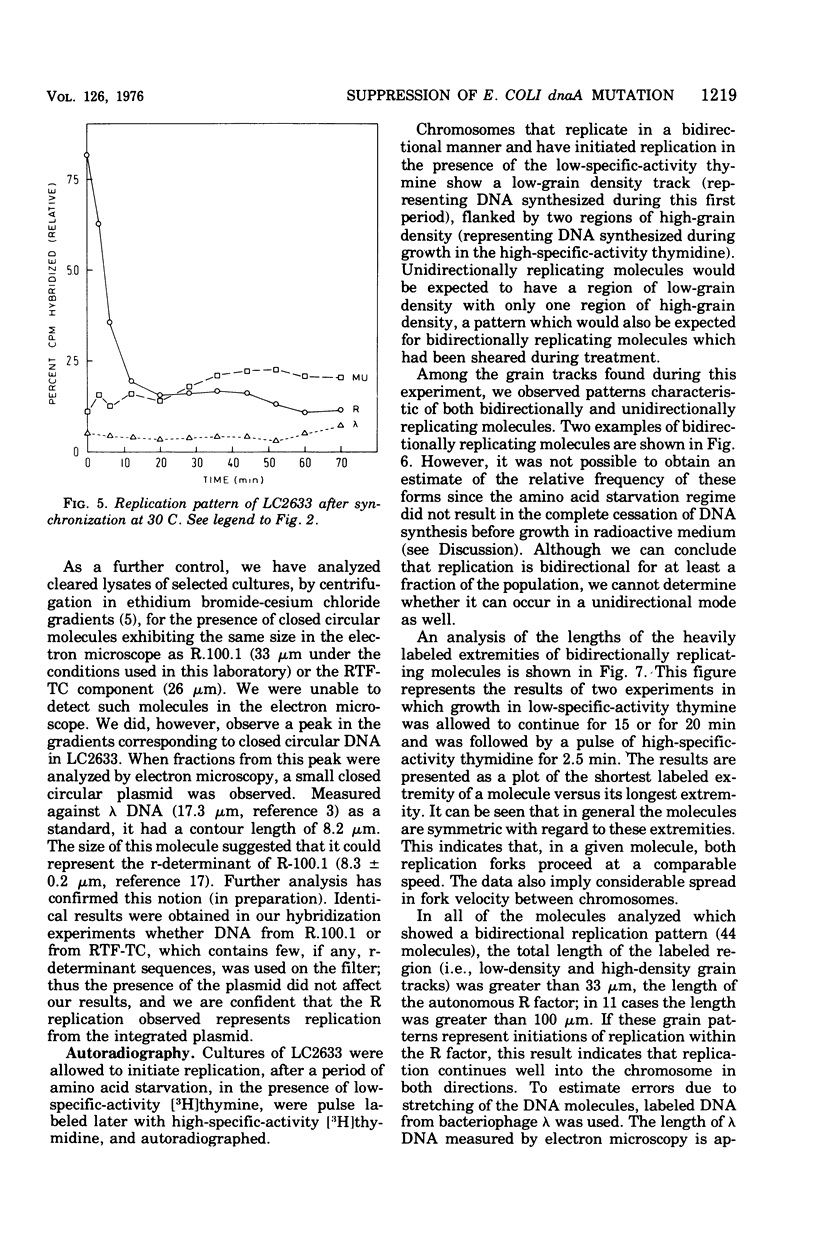
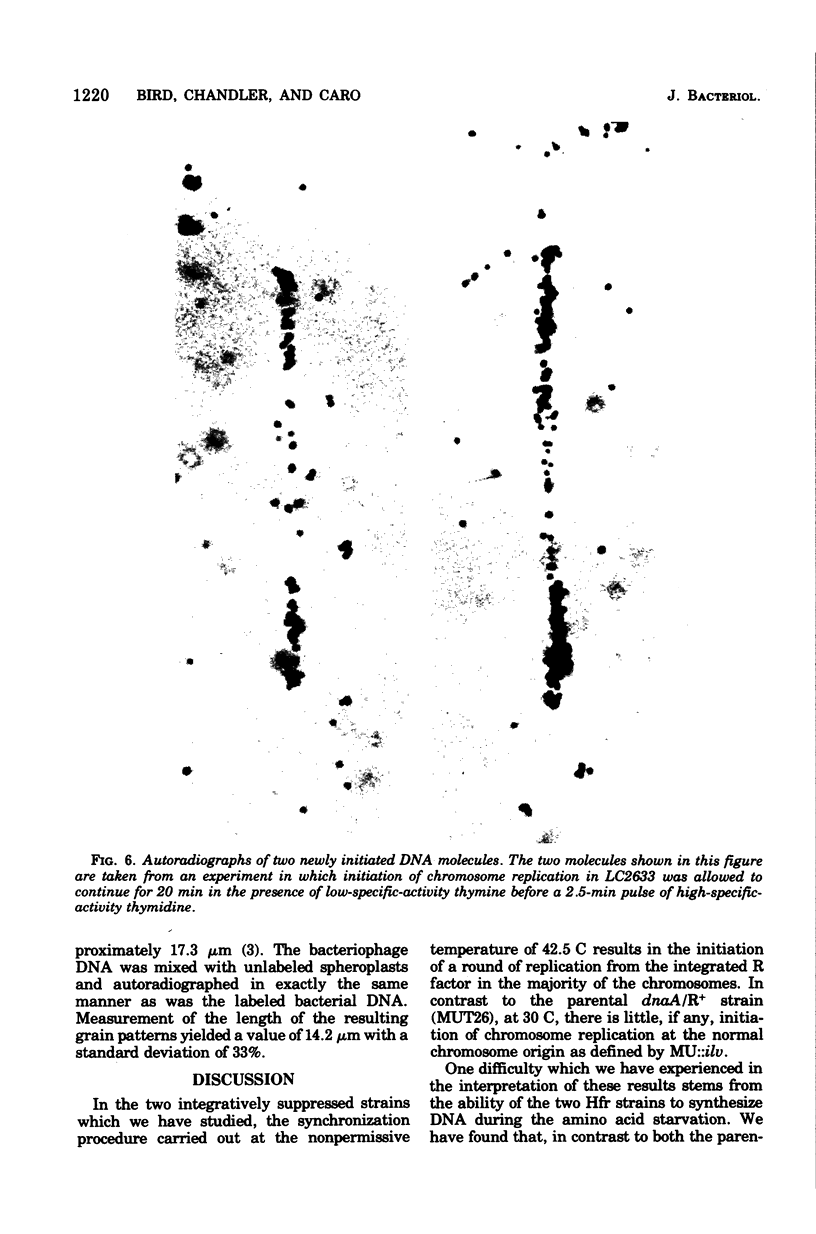
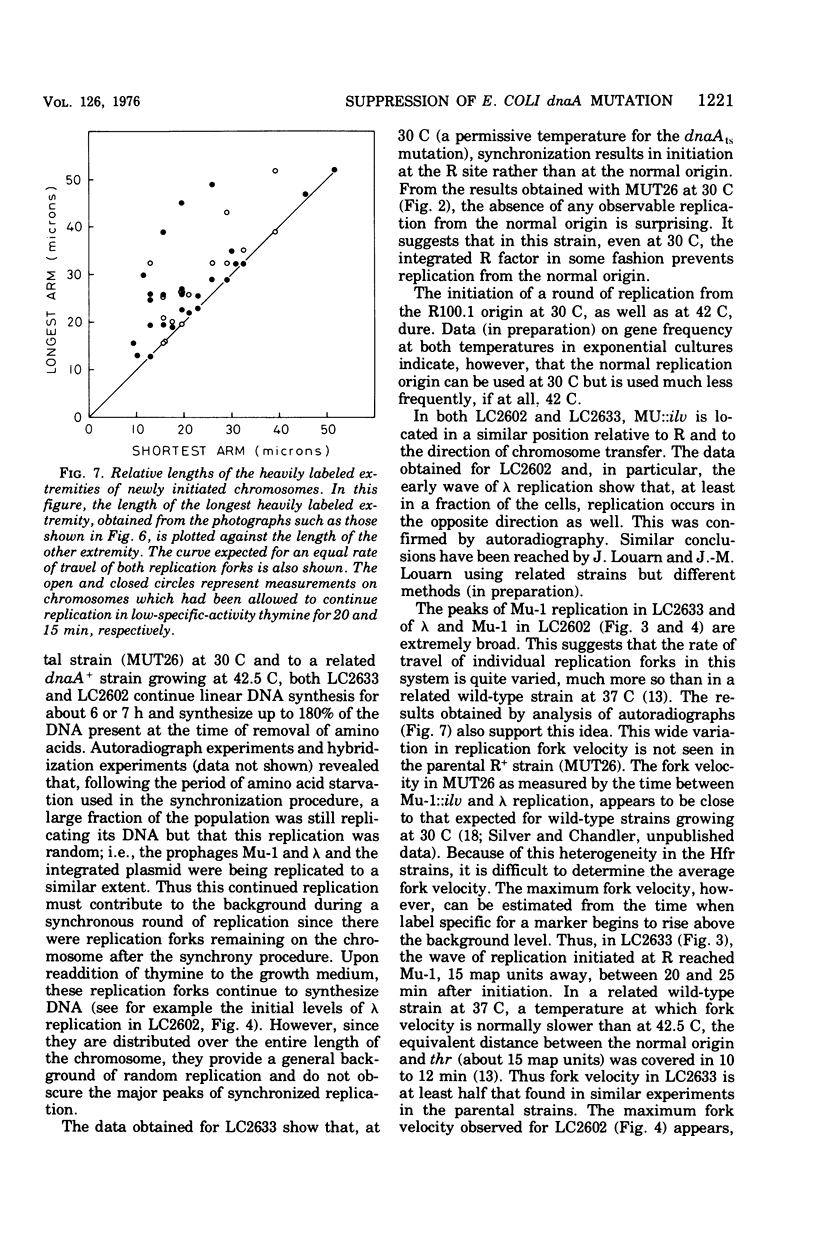


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- CARO L. G. THE MOLECULAR WEIGHT OF LAMBDA DNA. Virology. 1965 Feb;25:226–236. doi: 10.1016/0042-6822(65)90201-1. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Berg C. M. Chromosome replication in some strains of Escherichia coli K12. Cold Spring Harb Symp Quant Biol. 1968;33:559–573. doi: 10.1101/sqb.1968.033.01.063. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Leidner J., Tremblay G. Y. DNA-DNA hybridization on filters at low temperature in the presence of formamide or urea. Biochimie. 1971;53(10):1111–1114. doi: 10.1016/s0300-9084(71)80201-8. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L. Deoxyribonucleic acid-deoxyribonucleic acid hybridization assay for replication origin deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1972 Jun;110(3):917–925. doi: 10.1128/jb.110.3.917-925.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lark C. G., Consigli R., Toliver A. DNA replication in Chinese hamster cells: evidence for a single replication fork per replicon. J Mol Biol. 1971 Jun 28;58(3):873–875. doi: 10.1016/0022-2836(71)90046-5. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli: replication of the bacterial chromosome under control of prophage P2. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2407–2411. doi: 10.1073/pnas.68.10.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J., Funderburgh M., Bird R. E. More precise mapping of the replication origin in Escherichia coli K-12. J Bacteriol. 1974 Oct;120(1):1–5. doi: 10.1128/jb.120.1.1-5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Nishimura A., Nishimura Y., Caro L. Isolation of Hfr strains from R+ and ColV2+ strains of Escherichia coli and derivation of an R'lac factor by transduction. J Bacteriol. 1973 Dec;116(3):1107–1112. doi: 10.1128/jb.116.3.1107-1112.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M., Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J Mol Biol. 1971 Feb 14;55(3):441–456. doi: 10.1016/0022-2836(71)90328-7. [DOI] [PubMed] [Google Scholar]
- Perlman D., Twose T. M., Holland M. J., Rownd R. H. Denaturation mapping of R factor deoxyribonucleic acid. J Bacteriol. 1975 Sep;123(3):1035–1042. doi: 10.1128/jb.123.3.1035-1042.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci O. Chromosome replication and cell division in Escherichia coli at various temperatures of growth. J Bacteriol. 1972 Feb;109(2):848–854. doi: 10.1128/jb.109.2.848-854.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kuempel P. L. Bidirectional replication of the chromosome in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2842–2845. doi: 10.1073/pnas.69.10.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]