Abstract
Objective:
Anecdotal evidence suggests that use of Exercise Sandals results in a number of positive clinical outcomes. However, little research has been conducted to determine their efficacy objectively. Our purposes were to determine the effect of Exercise Sandals on lower leg electromyography (EMG) during activities in the Exercise Sandals and to compare EMG associated with Exercise Sandals with traditional lower extremity rehabilitation exercises.
Design and Setting:
Two within-subjects, repeated-measures designs were used to identify differences in lower extremity EMG: (1) between activities with and without Exercise Sandals and (2) between Exercise Sandals activities and traditional rehabilitation activities. All data were collected in the Sports Medicine Research Laboratory.
Subjects:
Eighteen subjects involved in rehabilitation using Exercise Sandals for at least 2 weeks within the year before data collection.
Measurements:
Mean EMG amplitudes from the tibialis anterior, peroneus longus, soleus, and lateral gastrocnemius muscles were measured during single-leg stance, side stepping, and “high knees,” all performed with and without the Exercise Sandals, as well as single-leg stance on a foam surface and T-band kicks in the sagittal and frontal planes.
Results:
Exercise Sandals increased lower leg EMG activity, particularly in the ankle invertors and evertors. Also, activities involving the Exercise Sandals resulted in EMG activity similar to or exceeding that associated with traditional ankle-rehabilitation exercises.
Conclusions:
These results, coupled with the fact that Exercise Sandals are used in a functional closed kinetic chain manner, suggest that they are an effective means of increasing lower extremity muscle activity.
Keywords: balance training, ankle rehabilitation, closed kinetic chain rehabilitation
A primary goal of rehabilitation is facilitating muscle activity to reestablish normal or enhanced neuromuscular control. Consequently, reestablishing neuromuscular control and enhancing dynamic stability are aimed at restoring functional joint stability. Previous researchers have demonstrated improvements in muscle activity,1 postural control,2–6 and proprioceptive capabilities3,7 and decreased incidence of subsequent injury8 with the use of various prophylactic, training, and rehabilitation devices and techniques. These implements include open kinetic chain strength training, athletic tape, foot orthotics, trampolines, wobble boards, and foam surfaces. However, most of these studies involved rehabilitation and training techniques that are performed in either static or open kinetic chain manners.3,5,8–11 Static rehabilitation activities and those performed in the open kinetic chain may not represent functional activity and may not accurately characterize the status of the lower extremity during the mechanism of the most common lower extremity injury, the inversion ankle sprain.
Exercise Sandals (Orthopedic Physical Therapy Products, Minneapolis, MN) allow lower extremity balance and strengthening exercises to be performed in a functional, closed kinetic chain manner. These rehabilitation devices consist of a cork sandal with a rubber sole and a rubber hemisphere similar in consistency to a lacrosse ball positioned under the midsole (Figure 1). The design of the sandals essentially creates an individualized perturbation device for each limb that can be used in any number of functional activities, ranging from static, single-leg stance (SLS) to dynamic gait activities. Exercise Sandals are introduced in the clinical setting in a progressive manner. The protocol developed in the clinical setting initially involves instruction in the short-foot concept,12 whereby the patient learns to contract the intrinsic foot flexors in the absence of toe flexion. Once the patient successfully learns the short-foot concept, gait tasks using the Exercise Sandals are introduced. These include side stepping (SS), forward walking, and heel kicks (in which the patient flexes the knee excessively, bringing the calcaneus toward the gluteal region). Sport-specific activities can also be included. The exercises then progress into “high knees” (HK) and more difficult tasks such as lunge walking. The final, most difficult stage of the progression is SLS in the Exercise Sandals (SLS-ES).
Figure 1.

Exercise Sandals.
Exercise Sandals have been used in the clinical setting for treatment of acute ankle sprains and chronic instability, anterior tibial compartment syndrome, and lower leg fractures and enhancing core stability. Bullock-Saxton et al13 demonstrated increased gluteal activity during walking using the “balance shoes.” To our knowledge, no other published research has been performed to objectively quantify the effects of “balance shoes” on various physiologic measures. The positive clinical outcomes observed by the athletic training staff with the use of the Exercise Sandals are anecdotal in nature; thus, the efficacy of the Exercise Sandals cannot be established directly from these observations. Therefore, our purposes were to determine the effect of the Exercise Sandals on activation of the lower leg musculature during a series of functional activities and to compare muscle activity associated with activities in the Exercise Sandals with traditional rehabilitation exercises advocated for increasing lower extremity muscle activity.
METHODS
All data collection occurred in a single session lasting approximately 90 minutes. Before participating, all subjects read and signed an informed consent document that had been previously approved by the Committee on the Protection of the Rights of Human Subjects at the University of North Carolina at Chapel Hill, which also approved the study.
Subjects
Eighteen individuals (10 men, 8 women) involved in rehabilitation using the Exercise Sandals for a minimum of 2 weeks within the year before data collection participated in this investigation (age = 21 ± 8 years, height = 1.73 ± 0.11 m, mass = 69.73 ± 13.04 kg). Subjects included Division I collegiate athletes participating in lacrosse (n = 5), fencing (n = 4), gymnastics (n = 2), soccer (n = 2), crew (n = 2), track (n = 1), and volleyball (n = 1), and 1 subject was a recreational triathlete. Prior experience with the Exercise Sandals was required as an inclusion factor to reduce the effect novel activity may have on electromyographic (EMG) signals.14,15 All subjects were also required to be pain free during all functional activities with and without the Exercise Sandals.
Data Collection
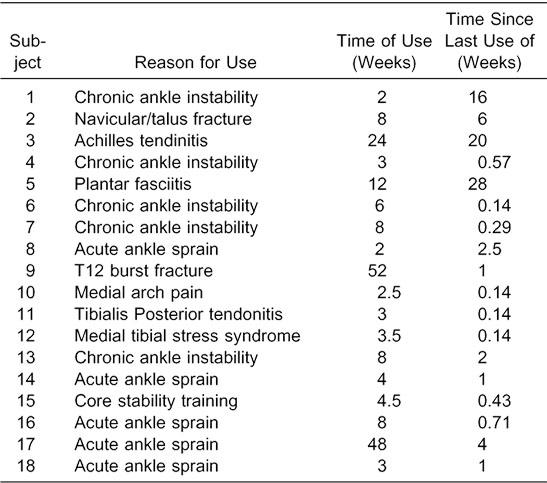
Before data collection, each subject completed a questionnaire detailing the reason for use of the Exercise Sandals, length of time he or she had used the Exercise Sandals, time since the last use of the Exercise Sandals, sport participation, and lower limb dominance. Mean time of use of the Exercise Sandals was 11.6 ± 14.9 weeks, and mean time since the last use of the Exercise Sandals was 4.9 ± 8.2 weeks. The injury for which each subject was involved in rehabilitation, time of use of the Exercise Sandals, and time since the use of the Exercise Sandals are provided in Table 1. The dominant limb was defined as the limb used to kick a ball, and all data were collected from this limb. After completing the questionnaire, subjects began a 5-minute warm-up period in the Exercise Sandals, during which they performed activities similar to those they had performed during rehabilitation.
Table 1.
Subject Injuries and Use of Exercise Sandals
Functional Activities
Data collection involved functional activities during which EMG signals were recorded from the tibialis anterior (TA), peroneus longus (PL), soleus (SOL), and lateral gastrocnemius (LG) muscles of the dominant leg. These activities included SLS, SS, and HK with and without the Exercise Sandals. During the HK conditions, subjects walked forward while flexing the hip of the nonstance leg to at least 90°. We also compared lower extremity EMG between SLS-ES and traditional lower extremity rehabilitation activities, including SLS on a foam surface (SLS-F), and T-band kicks in the frontal (TB-F) and sagittal (TB-S) planes. These traditional rehabilitation activities were chosen because they all involve SLS and are thought to increase the demands placed on the lower extremity musculature to provide postural stability.9,16
Single-leg–stance conditions were performed on the dominant leg with the eyes open and the knee and hip of the nondominant leg flexed to a comfortable position. Subjects were allowed to perform any necessary compensatory movements to maintain balance. We instructed subjects to assume a balanced position and to indicate verbally when ready to begin testing. After the subject's verbal cue, we initiated data collection, and the subject maintained the balanced position for 12 seconds.
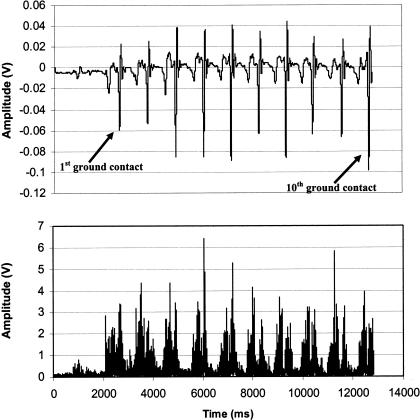
Side stepping and HK were performed in similar manners. Subjects completed 10 SS cycles and 10 HK cycles, providing 10 ground-contact episodes for the dominant leg. We identified ground contact by aligning the x-axis of a triaxial accelerometer (model 356A22, PCB Piezotronics Inc, Depew, NY) parallel to the tibial longitudinal axis and visually locating the point of ground contact in the accelerometer signal. To standardize influences of movement velocity on EMG responses, these activities were performed to a cadence set by a quartz metronome at 52 beats per minute. Subjects made ground contact with the dominant leg with each beat of the metronome for the SS conditions and with either foot in an alternating manner during HK conditions. Subjects performed 3 practice trials to become familiar with the cadence. After a verbal cue from the investigator, subjects performed 10 consecutive steps.
During T-band kicks, subjects stood on the dominant leg with gray (extra-heavy) Theraband tubing (Hygenic Corp, Akron, OH) tied around the nondominant leg and the opposite end of the tubing anchored to a treatment table. Subjects stepped away from the table so that a self-selected, comfortable amount of tension was developed in the Theraband. Subjects performed 20 kicking motions with the nondominant limb away from the table in either the sagittal or frontal plane such that resistance was created in the Theraband. These repetitive kicking motions created a balance perturbation to which the muscles of the stance (dominant) leg were forced to react.9 Movement velocity was controlled by setting the metronome to 112 beats per minute. Subjects initiated each kick with each beat of the metronome and performed 3 practice trials to become familiar with the cadence. After a verbal cue from the investigator, the subjects performed 20 consecutive kicks.
Subjects performed 3 trials for each activity, and testing order was counterbalanced to reduce the likelihood of an order effect. The likelihood of fatigue was reduced by allowing 30 seconds of rest between trials and 1 minute of rest between conditions.
Variables Assessment
We recorded signals using a telemetry EMG system (model T42L-8TO, Konigsberg Instruments, Inc, Pasadena, CA). This system consists of an 8-channel differential preamplifier/encoder/transmitter and a receiver/demodulator (input impedance = 200 kΩ, common mode rejection ratio >70 dB, signal-to-noise ratio >40 dB). Electromyography signals were amplified by a factor of 10 000 over a bandwidth of 0.01 to 2000 Hz and passed via an A/D converter (National Instruments Corp, Austin, TX), which sampled EMG and accelerometer data at 1000 Hz to a storage computer. Two self-adhesive surface electrodes (Ag/AgCl discs, 2-cm diameter) were positioned over the area of greatest muscle bulk of the LG, SOL, PL, and TA muscles with an interelectrode distance of 2 cm, and a single reference electrode was positioned over the tibial tuberosity. Electrode sites for the LG, PL, and TA were determined by having the subject contract the specific muscle, palpating the length of the muscle belly, and determining the area of greatest bulk. Electrodes for the SOL were positioned on the posterolateral tibia, inferior to the lower border of the belly of the LG. Electrode sites were prepared by shaving any hair from the area, abrading the skin, and cleansing the skin with isopropyl alcohol. Proper electrode placement and minimal cross-talk were verified by performing manual muscle tests17 and observing the output on an oscilloscope (model TDS 2014, Tektronix Inc, Beaverton, OR). To secure electrodes, we placed prewrap over the sites and anchored it with athletic tape. The accelerometer was fixed to the distal tibia by securing it to a tongue depressor with wax, coating the tongue depressor with skin adhesive, and placing prewrap and athletic tape over the tongue depressor. The electrode and accelerometer leads were also bound and secured to limit motion artifact. Subjects carried the telemeter in a holster secured to the body by a belt to allow for functional movements. All data were collected using custom LabVIEW software (version 6i, National Instruments Corp).
Data Reduction
All EMG and accelerometer data were reduced using the custom LabVIEW software. After A/D conversion, EMG signals were corrected for DC bias; bandpass (10–350 Hz) and notch (59.5–60.5 Hz) filtered using fourth-order, zero-phase log, Butterworth filters; and full-wave rectified. Accelerometer signals were also low-pass filtered at 10 Hz using a fourth-order, zero-phase log, Butterworth filter to eliminate motion artifact and to smooth the data to provide a clearer representation of the instant at which ground contact occurred.
For SLS conditions, we used the middle 10 seconds of each 12-second trial for analysis. Mean amplitude of the EMG signal (mEMG) in volts for each muscle was calculated over this 10-second interval. For SS and HK conditions, mEMG was calculated for each muscle over the entirety of the activity, from the first ground contact episode to the 10th ground-contact episode identified by the accelerometer (Figure 2). The time interval over which these calculations were performed was relatively consistent (±1 second) across trials due to the use of the metronome. For T-band kicks, mEMG was calculated over the middle 10 seconds of each trial. Subjects performed 20 kicks at a cadence of 112 beats per minute, allowing for a total trial time of approximately 11 seconds (20 beats × [1 min/112 beats] × [60 s/min] = 10.71 seconds).
Figure 2.
Typical accelerometer signal and associated tibialis anterior electromyography (EMG) during side stepping. The top graph represents acceleration of the lower limb, and the bottom graph represents tibialis anterior EMG activity. These data represent 1 subject during 1 trial.
Statistical Analyses
We performed all statistical analyses using SPSS (version 11.0, SPSS Inc, Chicago, IL). A series of activity (3 levels: SLS, SS, HK) × surface (2 levels: Exercise Sandals, no Exercise Sandals) repeated-measures analyses of variance (ANOVAs) were employed (1 for each muscle) to identify significant mean differences in mEMG. A separate repeated-measures ANOVA (4 levels: SLS-ES, SLS-F, TB-S, TB-F) was calculated for each muscle to identify significant mean differences between SLS activities involving the Exercise Sandals and traditional rehabilitation exercises. We used a Dunn-Bonferroni planned pairwise contrast procedure to determine specifically where significant differences were located. Statistical significance was established a priori at α = .05.
RESULTS
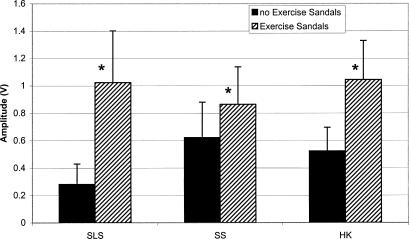
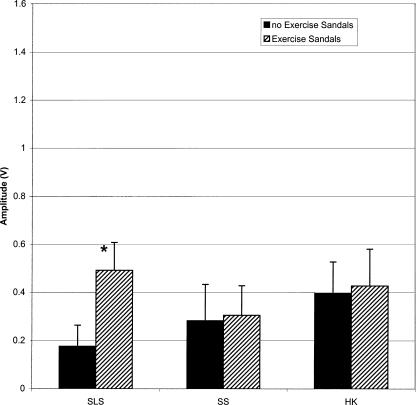
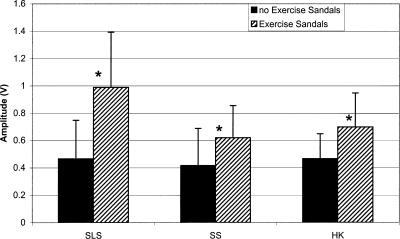
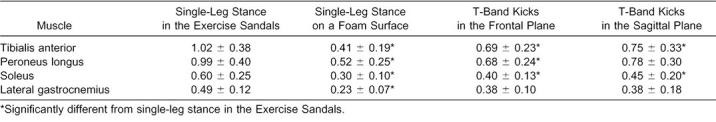
Each of the 3 × 2 ANOVAs revealed a significant activity × surface interaction (F3,51 = 21.887, P < .001; 16.689, P < .001; 19.247, P < .001; 34.105, P < .001 for the TA, PL, SOL, and LG, respectively). Dunn-Bonferroni planned pairwise contrasts indicated that mEMG for the TA, PL, and SOL was significantly greater for all activities with the Exercise Sandals compared with the same activities without the Exercise Sandals. Only SLS increased mEMG of the LG when performed in the Exercise Sandals compared with the same condition without the Exercise Sandals (Figures 3–6).
Figure 3.
Tibialis anterior (TA) mean electromyographic amplified (mEMG) for activities with and without Exercise Sandals. *Significantly different mEMG compared with the same activity performed without the Exercise Sandals. SLS indicates single-leg stance; SS, side stepping; HK, high knees.
Figure 6.
Lateral gastrocnemius (LG) mean electromyographic amplified (mEMG) for activities with and without Exercise Sandals. *Significantly different mEMG compared with the same activity performed without the Exercise Sandals. SLS indicates single-leg stance; SS, side stepping; HK, high knees.
Figure 4.
Peroneus longus (PL) mean electromyographic amplified (mEMG) for activities with and without Exercise Sandals. *Significantly different mEMG compared with the same activity performed without the Exercise Sandals. SLS indicates single-leg stance; SS, side stepping; HK, high knees.
Figure 5.
Soleus (SOL) mean electromyographic amplified (mEMG) for activities with and without Exercise Sandals. *Significantly different mEMG compared with the same activity performed without the Exercise Sandals. SLS indicates single-leg stance; SS, side stepping; HK, high knees.
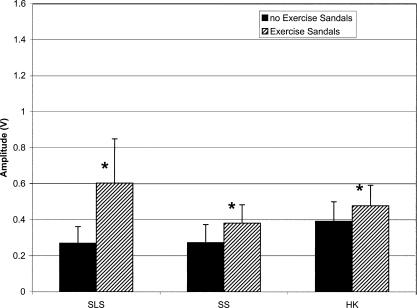
Each of the ANOVAs for comparisons of SLS activities involving the Exercise Sandals and traditional rehabilitation exercises revealed a significant main effect for activity (F6,102 = 27.288, P < .001; 26.318, P < .001; 18.694, P < .001; 18.003, P < .001 for the TA, PL, SOL, and LG, respectively). Dunn-Bonferroni planned pairwise contrasts within these analyses indicated that SLS-ES resulted in significantly greater mEMG for all muscles compared with that resulting from SLS-F. The SLS-ES also resulted in significantly greater mEMG in the TA, PL, and SOL compared with TB-F and in the TA and SOL compared with TB-S. Mean values and standard deviations for each muscle during each SLS activity involving the Exercise Sandals and traditional rehabilitation exercises are provided in Table 2.
Table 2.
Amplitude (V) of Exercise Sandals Activities With Traditional Rehabilitation Activities (Mean ± SD)
DISCUSSION
Our results reflect increased muscle activity in the lower leg during activities performed with the Exercise Sandals compared to the same activities performed without the Exercise Sandals. Additionally, SLS-ES produced mEMG activity equal to or exceeding that of traditional lower extremity rehabilitation exercises that involve SLS. Thus the Exercise Sandals are an effective device for increasing lower extremity muscle activity.
The EMG activity of the TA, SOL, and PL was greater during all Exercise Sandals activities compared with the same activities without the Exercise Sandals. Similarly, EMG activity of these muscles during SLS-ES was greater than that occurring during traditional rehabilitation activities that involve SLS. Although the anterior and posterior tibialis muscles are considered the primary ankle invertors, the SOL also functions as an invertor.18–20 These results suggest that the Exercise Sandals increase functional demands placed on the ankle invertors and evertors. Ankle sprains, particularly inversion sprains, are one of the most common injuries to the lower extremity.21–23 As such, a large percentage of lower extremity rehabilitation involving strengthening, range of motion, and proprioception is highly concentrated on these muscles. Our results suggest that the Exercise Sandals provide a challenge to this musculature, indicating that the Exercise Sandals may be an effective rehabilitation tool for neuromuscular rehabilitation of the lower extremity.
A secondary goal of this investigation was to determine the efficacy of the Exercise Sandals in eliciting muscle responses compared with activities that have been traditionally used in the athletic training setting to increase postural-stability demands and lower extremity muscle activity. To achieve this goal, subjects performed SLS on a foam surface and T-band kicks in the frontal and sagittal planes as described by Tomaszewski.9 We then compared mean EMG between these activities and SLS-ES, as each activity involved SLS on the dominant limb.
Single-leg stance in the Exercise Sandals resulted in significantly greater mEMG in all muscles compared with SLS-F. Single-leg stance on unstable surfaces such as foam, wobble boards, and trampolines is thought to alter somatosensory information16 and has been advocated for increasing the functional demands placed on the musculoskeletal and sensorimotor systems during lower extremity rehabilitation.3,5,8,10,11 Our results suggest that the Exercise Sandals may be more effective than SLS on firm or foam surfaces for attaining this goal because EMG of muscles acting in both the frontal and sagittal planes displayed at least a twofold increase with use of the Exercise Sandals.
T-band kicks are widely used in the athletic training setting following lower extremity injury, particularly ankle injury. Compared with mEMG of the stance leg during TB-F, SLS-ES resulted in greater activity of the TA, PL, and SOL. Similarly, SLS-ES resulted in greater mEMG of the TA and SOL compared with the stance leg during TB-S. These results suggest that SLS-ES is more effective in recruiting lower extremity muscle activity than the traditional rehabilitation activities used in this investigation and, therefore, may be more effective in promoting restoration of normal function to muscles affected by injury.
Noncontact injury to the ankle generally occurs upon ground contact after a closed kinetic chain mechanism of forced inversion and plantar flexion.1,8,24 Previous research suggests that performing activities in a closed kinetic chain may increase afferent input to the sensorimotor system by stimulating cutaneous, joint, and ligament mechanoreceptors,25–27 allowing for enhanced proprioceptive capabilities. Cordova et al1 suggested that closed kinetic chain exercises are advantageous for lower extremity rehabilitation because they result in cocontraction of various muscle groups about the ankle joint, thus simulating functional activity and allowing for sport-specific rehabilitation. These authors also noted that exercises resulting in greater muscle activity may be the most efficient for promoting muscle-strength gains and reestablishing neuromuscular control, emphasizing the importance of closed kinetic chain rehabilitation in the lower extremity. The Exercise Sandals exert their effects on lower extremity muscle activation during functional activities similar to gait. By allowing for increased muscle activity in a more functional, closed kinetic chain position, return to normal muscle function may be enhanced or expedited (or both) through use of the Exercise Sandals.
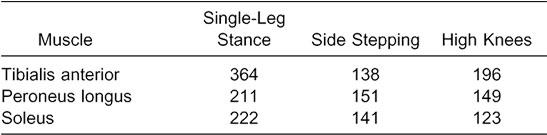
Increased muscle activity may not be a positive outcome for every injury scenario. For example, increased ankle-invertor strength without concomitant increases in evertor strength may predispose the ankle joint to inversion injury after invertor-evertor coactivation. With this concept in mind, we analyzed the percentage increase in mEMG of the TA and SOL relative to the PL was analyzed (Table 3). These data indicated that SLS-ES and HK-ES resulted in much larger percentage increases in TA activity relative to the PL, whereas increases in the SOL were similar to the PL. The relative increases for each muscle during SS were similar in all 3 muscles. Therefore, these results should be applied cautiously to the clinical setting, in that the level of agonist-antagonist cocontraction may be of greater importance for evaluation of muscle function compared with activity of isolated muscles. Future research is necessary to determine the effects of this increased muscle activity on the injury-recovery process.
Table 3.
Percentage Increases in Invertor-Evertor mEMG Activity With Use of the Exercise Sandals
Although little research has been conducted to determine the means by which the Exercise Sandals are an effective rehabilitation tool in the clinical setting, our results provide insight into the neuromuscular response of the lower leg to the Exercise Sandals. We have demonstrated that the Exercise Sandals effectively increased EMG activity in the lower leg and that activities performed in the Exercise Sandals resulted in EMG activity greater than or equal to that associated with activities traditionally used in lower extremity rehabilitation in the athletic training setting to increase muscle activity. These findings provide objective information that lends justification to the use of the Exercise Sandals in the clinical setting to promote lower extremity muscle activity. However, future research is necessary to determine the effect of the Exercise Sandals on various clinical-outcome measures such as postural stability and proprioceptive capability in functionally unstable ankles.
REFERENCES
- 1.Cordova ML, Jutte LS, Hopkins JT. EMG comparison of selected ankle rehabilitation exercises. J Sport Rehabil. 1999;8:209–218. [Google Scholar]
- 2.Hoffman M, Payne VG. The effects of proprioceptive ankle disk training on healthy subjects. J Orthop Sports Phys Ther. 1995;21:90–93. doi: 10.2519/jospt.1995.21.2.90. [DOI] [PubMed] [Google Scholar]
- 3.Bernier JN, Perrin DH. Effect of coordination training on proprioception of the functionally unstable ankle. J Orthop Sports Phys Ther. 1998;27:264–275. doi: 10.2519/jospt.1998.27.4.264. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn JT, Guskiewicz KM, Busby MA, Prentice WE. Balance and joint stability: the relative contributions of proprioception and muscular strength. J Sport Rehabil. 2000;9:315–328. [Google Scholar]
- 5.Gauffin H, Tropp H. Altered movement and muscular-activation patterns during the one-legged jump in patients with an old anterior cruciate ligament rupture. Am J Sports Med. 1992;20:182–192. doi: 10.1177/036354659202000215. [DOI] [PubMed] [Google Scholar]
- 6.Guskiewicz KM, Perrin DH. Effect of orthotics on postural sway following inversion ankle sprain. J Orthop Sports Phys Ther. 1996;23:326–331. doi: 10.2519/jospt.1996.23.5.326. [DOI] [PubMed] [Google Scholar]
- 7.Robbins S, Waked E, Rappel R. Ankle taping improves proprioception before and after exercise in young men. Br J Sports Med. 1995;29:242–247. doi: 10.1136/bjsm.29.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wester JU, Jespersen SM, Nielsen KD, Neumann L. Wobble board training after partial sprains of the lateral ligaments of the ankle: a prospective randomized study. J Orthop Sports Phys Ther. 1996;23:332–336. doi: 10.2519/jospt.1996.23.5.332. [DOI] [PubMed] [Google Scholar]
- 9.Tomaszewski D. T-band kicks ankle proprioception program. Athl Train J Natl Athl Train Assoc. 1991;26:216–219. [Google Scholar]
- 10.Irrgang JJ, Whitney SL, Cox ED. Balance and proprioceptive training of the lower extremity. J Sport Rehabil. 1994;3:68–83. [Google Scholar]
- 11.Cox ED, Lephart SM, Irrgang JJ. Unilateral balance training of noninjured individuals and the effects on postural sway. J Sport Rehabil. 1993;2:87–96. [Google Scholar]
- 12.Janda V, Va'Vrona M. Sensory motor stimulation. In: Liebenson C, editor. Rehabilitation of the Spine: A Practitioner's Manual. New York, NY: Lippincott Williams & Wilkins; 1995. pp. 322–323. [Google Scholar]
- 13.Bullock-Saxton JE, Janda V, Bullock MI. Reflex activation of gluteal muscle in walking: an approach to restoration of muscle function for patients with low-back pain. Spine. 1993;18:704–708. [PubMed] [Google Scholar]
- 14.Flament D, Shapiro MB, Kempf T, Corcos DM. Time course and temporal order of changes in movement kinematics during learning of fast and accurate elbow flexions. Exp Brain Res. 1999;129:441–450. doi: 10.1007/s002210050911. [DOI] [PubMed] [Google Scholar]
- 15.Spencer JP, Thelen E. A multimuscle state analysis of adult motor learning. Exp Brain Res. 1999;128:505–516. doi: 10.1007/s002210050873. [DOI] [PubMed] [Google Scholar]
- 16.Guskiewicz KM, Perrin DH. Research and clinical applications of assessing balance. J Sport Rehabil. 1996;5:45–63. [Google Scholar]
- 17.Hislop HJ, Montgomery J. Daniels and Worthingham's Muscle Testing: Techniques of Manual Examination. Vol 6. Philadelphia, PA: WB Saunders; 1995. [Google Scholar]
- 18.Stormont DM, Morrey DM, An KM, Cass JR. Stability of the loaded ankle joint: relation between articular restraint and primary and secondary static restraints. Am J Sports Med. 1983;13:295–300. doi: 10.1177/036354658501300502. [DOI] [PubMed] [Google Scholar]
- 19.Klein P, Mattys S, Rooze M. Moment arm length variations of selected muscles acting on talocrural and subtalar joints during movement: an in vitro study. J Biomech. 1996;29:21–30. doi: 10.1016/0021-9290(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 20.Hintermann B, Nigg BM, Sommer C. Foot movement and tendon excursion: an in vitro study. Foot Ankle Int. 1994;15:386–395. doi: 10.1177/107110079401500708. [DOI] [PubMed] [Google Scholar]
- 21.Garrick JG. The frequency of injury, mechanisms of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977;5:241–242. doi: 10.1177/036354657700500606. [DOI] [PubMed] [Google Scholar]
- 22.Kannus P, Renstrom P. Treatment for acute tears of the lateral ligaments of the ankle: operation, cast, or early controlled mobilization. J Bone Joint Surg Am. 1991;73:305–312. [PubMed] [Google Scholar]
- 23.Balduini FC, Tetzlaff J. Historical perspectives on injuries of the ligaments of the ankle. Clin Sports Med. 1982;1:3–12. [PubMed] [Google Scholar]
- 24.Ekstrand J, Tropp H. The incidence of ankle sprains in soccer. Foot Ankle. 1990;11:41–44. doi: 10.1177/107110079001100108. [DOI] [PubMed] [Google Scholar]
- 25.Snyder-Mackler L. Scientific rationale and physiological basis for the use of closed kinetic chain exercises in the lower extremity. J Sport Rehabil. 1996;5:2–12. [Google Scholar]
- 26.Birmingham TB, Kramer JF, Inglis JT, et al. Effect of a neoprene sleeve on knee joint position sense during sitting open kinetic chain and supine closed kinetic chain tests. Am J Sports Med. 1998;26:562–566. doi: 10.1177/03635465980260041601. [DOI] [PubMed] [Google Scholar]
- 27.Andersen SB, Terwilliger DM, Denegar CR. Comparison of open versus closed kinetic chain test positions for measuring joint position sense. J Sport Rehabil. 1995;4:165–171. [Google Scholar]