Abstract
Objective:
Although cryotherapy and high-voltage electric stimulation, both alone and in combination, are commonly applied to curb acute edema, little evidence from randomized controlled studies supports these procedures. Our purpose was to examine the effects of cool-water immersion (CWI) at 12.8°C (55°F), cathodal high-voltage pulsed current (CHVPC) at 120 pulses per second and 90% of visible motor threshold, and the combination of CWI and CHVPC (CWI + CHVPC) on edema formation after impact injury to the hind limbs of rats.
Design and Setting:
Both feet of 34 rats were traumatized after hind-limb volumes were determined. Animals were randomly assigned to 1 of 3 groups: CWI (n = 10), CHVPC (n = 10), or CWI + CHVPC (n = 14). One randomly selected hind limb of each rat was exposed to four 30-minute treatments, interspersed with four 30-minute rest periods beginning immediately after posttraumatic limb volumes were determined. Contralateral limbs served as controls. Limbs remained dependent during all treatments, rest periods, and volumetric measurements.
Subjects:
We used 34 anesthetized Zucker Lean rats in this study.
Measurements:
We measured limb volumes immediately before and after trauma and after each of 4 treatment and rest periods.
Results:
Volumes of treated limbs of all 3 experimental groups were smaller (P < .05) than those of untreated limbs. No treatment was more effective than another.
Conclusions:
Cool-water immersion, cathodal high-voltage electric stimulation, and simultaneous application of these treatments were effective in curbing edema after blunt injury. Combining CWI and CHVPC was not more effective than either CWI or CHVPC alone.
Keywords: cryotherapy, electrotherapy, swelling, animal model
Athletic trainers, physical therapists, and other health care professionals have long advocated the use of cryotherapy and electric stimulation to curb edema formation after orthopaedic injuries. Edema, a natural part of the inflammatory process, is initiated by virtually any trauma, including athletic injury. However, uncontrolled edema can increase pain, prolong immobilization, reduce range of joint motion, and inhibit ligament healing, all of which may extend time to recovery.1,2 Cryotherapy, in conjunction with compression and elevation, is almost universally accepted as an effective method of controlling edema after acute athletic trauma. Cryotherapy is thought to curb acute edema formation by decreasing blood flow,3,4 metabolic activity,5,6 and permeability of postcapillary venules.7 An earlier controlled trial from our laboratory indicated that immersing rat limbs in 12.8°C to 15.6°C (55–60°F) water immediately after blunt injury effectively curbed edema formation.8 In addition to cryotherapy, elevation, and compression, clinicians often use other modalities such as electric stimulation to curb acute edema formation.9 However, the efficacy of this last modality remains clinically unsubstantiated. Our laboratory established that high-voltage cathodal stimulation at 120 pulses per second (PPS) and 90% of visible motor threshold curbed edema formation in rats and frogs.10–12 Work by Reed,13 Taylor et al,14 and Karnes et al15 indicated that high-voltage electric stimulation curbs acute edema formation by decreasing permeability of microvessels.
If cryotherapy and high-voltage electric stimulation curb edema by affecting different root causes, then applying them simultaneously might produce a greater treatment effect than applying them individually. We were unaware of any randomized, controlled studies designed to examine the effects of simultaneous application of cryotherapy and high-voltage electric stimulation on acute edema formation. Therefore, our purposes were to (1) reexamine the effects of cool-water immersion and electric stimulation individually, and (2) determine the effects of combining cool-water immersion and electric stimulation on acute edema formation after blunt trauma to rats. Because human trials are difficult to control, we designed mock clinical trials with rats as subjects. Using rats allowed us to control age, sex, size, degree, and location of injury. In addition, effects of drugs or placebo were eliminated.
METHODS
Subjects
We used 38 Zucker Lean rats (Harlan Sprague Dawley Inc, Indianapolis, IN) in this study. Because we did not measure pretrauma limb volumes twice (to ensure accuracy) on the first 4 animals we processed, we excluded them from our data set. The remaining 34 rats, weighing 251 to 340 g (mean = 284 ± 2 g) were used for analysis. Animals were provided food and water ad libitum until used. The Institutional Laboratory Animal Care Committee of the State University of New York at Buffalo approved anesthesia and handling procedures, including mode of traumatizing hind limbs and sacrifice. We randomly assigned each subject to 1 of 3 treatment groups.
Instrumentation and Procedures
We induced impact injury by a procedure similar to that described by Mendel et al.12 This consisted of dropping a steel rod weighing 85.5 g through a vertical tube from a height of 30 cm onto the plantar aspect of each hind foot just distal to the malleoli. A rectangular piece of plastic (2 cm × 2 cm × 0.5 cm) was interposed between the foot and the tube to distribute the force of impact. This method of inducing trauma resulted in changes in limb volume that were attributable to edema formation and not frank bleeding (ie, it caused tissue damage without rupturing major vessels).8,11,12,16 Furthermore, the skin on these rats was translucent, and we observed no change in color throughout the procedures.
Limb Volume Measurement
We determined limb volumes by immersing a hind limb and measuring the amount of water displaced (see Dolan et al8 for a figure of the measurement system). The immersion vessel was 2 cm in diameter and 6 cm long. The inferior end of the vessel was tapered and an additional 6 cm long. A 3-way stopcock was attached so that the vessel could be rapidly filled through the tapered end of the vessel. A 23-gauge stainless-steel tube, 3-cm long with a 90° bend in the middle, was affixed with epoxy to the inside wall so that 1.5 cm of the tube extended into the immersion vessel. We attached the end of the tube outside the vessel to a 5-mL syringe via polyethylene tubing and a 23-gauge needle. Using this tubing complex as a siphon, the water level in the immersion vessel could be brought to the same level repeatedly. At the exact level as the tip of the stainless steel tube in the vessel, a 3-cm piece of 2-O thread was affixed with white plastic tape to the outside surface. We prepared the rats, suspended in cloth slings, for volume measurement by painting lines at the level of their malleoli. We then lowered the rats by motorized booms until the lines painted on their hind limbs were level with the threads affixed to the immersion vessels (at the level of the tips of the stainless-steel tubes). Displaced water was collected in 5-mL syringes by siphoning and weighed on an S-300D Micro Balance (Fisher Scientific, Pittsburgh, PA). The weight of the fluid collected was equivalent to the rat's limb volume (1 mL = 1 mg).
Reliability and Validity of Measurement System
Before initiating experiments, we established the reliability and validity of the volume-measurement system by determining the volume displacement of a small aluminum cylinder (1.270-cm diameter by 3.160-cm length). Use of an inanimate object to determine reliability and validity was deemed more appropriate than using volume measurements from animals because it has been shown that in dependent position, volumes of nontraumatized limbs change over time.17 Mean cylinder volume as determined from 10 consecutive measurements was 3.965 mL (SE = 0.013 mL, range = 3.915–4.005 mL). True volume of the cylinder calculated from physical dimensions (see above) was 4.003 mL. This is 0.038 mL greater than the volume of the cylinder as measured with our method, representing an underestimation of cylinder volume by 0.09% by our measuring method. Thus, our method of determining volume displacement is reliable and valid.
We lowered a rat via a motorized boom until its hind limbs were immersed to the painted lines in 100-mL beakers of water. Water in the treatment beakers was maintained at 12.8°C (55°F) for rats that received cryotherapy or cryotherapy and electric stimulation. Water for cathodal high-voltage pulsed current (CHVPC) and all control limbs was maintained at room temperature, 23°C (75°F). We selected this temperature range because Matsen et al18 reported that it had no therapeutic effect. Unpublished work from our laboratory confirmed this finding.
Body Temperature
Because anesthesia can cause body temperature to fall,19 we regulated body temperature throughout these experiments. A rectal probe was inserted 2 to 3 cm and connected to a YSI Tele-Thermometer (Yellow Springs Instruments Co, Yellow Springs, OH), and body temperature was monitored and recorded every 30 minutes throughout the experiment. Body temperature was regulated between 35°C and 37.5°C (95°F and 99.5°F) by directing a 60-W lamp either toward or away from a rat. Temperature of the cool-water beaker was also monitored continuously with a YSI Tele-Thermometer and probe. Shaved ice chips were added to beakers to maintain the desired temperature.
Experimental Protocol
Each rat was anesthetized by an intraperitoneal injection of sodium pentobarbital (65 mg/kg of body weight) and supplemented over the course of the 4-hour experiment as needed with doses of one half the original dose. Animals in the cool-water immersion (CWI) group needed an average of 1.6 boosters during the experiment (range = 1–3, n = 10), as did the animals in the CHVPC group (range = 1–3, n = 10), and those in the CWI + CHVPC group needed an average of 1.9 boosters (range = 1–3, n = 14). After the rats were anesthetized, we shaved their legs, feet, and abdomens. The rats were then placed in cloth slings and suspended at 45° (caudal end down) with both hind limbs fully exposed and in a dependent position. Lines were painted at the level of the malleoli, and the rectal probes were inserted.
After the limbs were suspended for approximately 20 minutes, we determined the volume (pretrauma) of each hind limb at least twice and averaged the values to determine the measurement. Both hind limbs of each rat were then injured by dropping a steel rod onto the plantar aspect of each foot just distal to the malleoli. Volumes of both hind limbs were again measured and, within 5 minutes after injury, the limbs were immersed in separate 100-mL beakers. The rats were randomly assigned to 1 of the 3 experimental treatments: CWI, CHVPC, or CWI + CHVPC. Treated limbs of animals receiving CWI or CWI + CHVPC were immersed in water maintained at 12.8°C (55°F). Rats receiving only CHVPC and all control limbs were immersed in water maintained at 23°C (73.4°F). As in previous work,10–12 cathodal high-voltage electric stimulation was applied at 120 PPS and 90% of visible motor threshold via the immersion technique. Cathodal high-voltage pulsed current was delivered via an Intelect 500S stimulator (Chattanooga Corp, Chattanooga, TN). Output consisted of twin-spiked, monophasic, pulsed current. Spikes of 5 and 8 microseconds were separated by an interphase interval of 75 microseconds. These pulses were delivered at 120 PPS for 30 consecutive minutes. Cloth slings used to suspend the rats were lined with 9-cm × 3.6-cm carbon-rubber electrodes (1 per sling), which functioned as anodes; these electrodes were coated with electrode gel and applied to shaved abdominal walls. Carbon-rubber electrodes were immersed in treatment beakers (1 per beaker), so that the water in the beakers served as cathodes. Animals in the CWI + CHVPC group received simultaneous application per the techniques described above.
Each animal received four 30-minute treatments interspersed with 30-minute rests. We determined the volume of each limb immediately after each treatment or rest period. Throughout treatments, rests, and measurements, animals remained hanging in slings with their limbs in dependent position. After removal from water beakers and before volume measurement, we dabbed the limbs with tissue paper to remove adherent water and to minimize evaporative cooling. At no time were limbs rubbed or squeezed during drying. At the conclusion of the experiment, animals were sacrificed by exposure to carbon dioxide.
Data Analysis
Data were expressed as changes from pretrauma hind-limb volumes per kilogram of body weight to minimize the effects of size on amount of swelling. We calculated a 3 × 2 × 9 (treatment group [CWI, CHVPC, CWI + CHVPC] × limb [treated, untreated] × time [0, 30, 60, 90, 120, 150, 180, 210, 240 minutes]) analysis of variance using SPSS 10.1 statistical software (SPSS Inc, Chicago, IL). Bonferroni-Holm post hoc procedures were used to determine significant comparisons. A P < .05 level of significance was set a priori for all analyses.
RESULTS
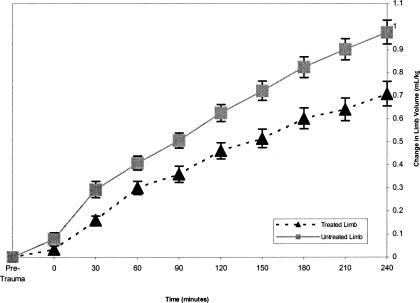
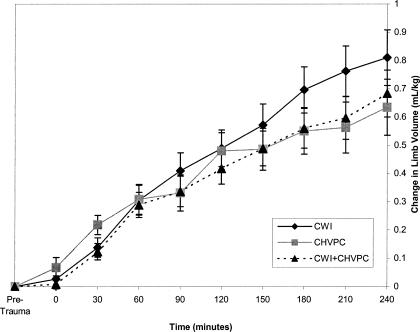
Volumes of treated limbs were less than those of untreated limbs over all times (F1,31 = 20.12, P = .001) (Figure 1, Table). However, no one treatment (CWI, CHVPC, or CWI + CHVPC) was more effective (F2,31 = 1.17, P = .325) than another (Figure 2). The Table presents the means and standard deviations for both treated and untreated limbs at each time interval from 30 to 240 minutes of the experiment. A large effect size was noted for all time periods except 60 minutes, which was classified as moderate.
Figure 1.
Treated-limb volumes for all 3 conditions (cool-water immersion, cathodal high-voltage pulsed current, and cool-water immersion plus cathodal high-voltage pulsed current) were smaller than untreated limbs (P < .05).
Effect-Size Calculations for Each Time Interval
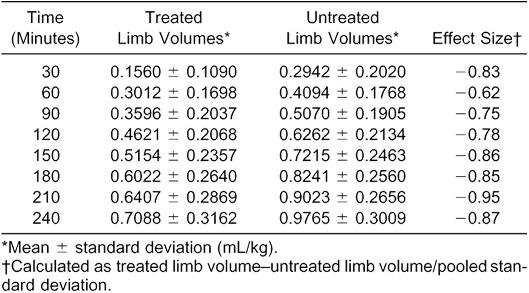
Figure 2.
Limb volumes across time, treated limbs only. Limb volumes across time did not differ among the 3 treatments (P < .05). CWI indicates cool-water immersion; CHVPC, cathodal high-voltage pulsed current, CWI + CHVPC, cool-water immersion plus cathodal high-voltage pulsed current.
DISCUSSION
Cool-water immersion (12.8°C [55°F]), cathodal high-voltage electric stimulation at 120 PPS and 90% of visible motor threshold, and simultaneous application of these modalities curbed acute edema formation, although the limbs were in dependent position throughout treatments, rest periods, and volumetric measurements. We maintained limbs in dependent position throughout the experiment because any other position could be construed as therapeutic and influence limb volumes. In addition, we chose to keep the rats anesthetized throughout data collection because we wanted to eliminate pain, stress, and sympathetic activity. Anesthesia also eliminated any exercise effect produced by muscle activity (ie, muscle pump) or behavior (such as rubbing or licking) that might influence limb volumes.
Our results corroborated the positive treatment effects observed in previous works on the effects of CWI8 and cathodal high-voltage electric stimulation.10–12,16,20 We hypothesized that combining these 2 treatment modalities might have an additive effect in curbing acute edema formation, but we observed no such effect.
Cryotherapy is thought to curb acute edema formation by decreasing blood flow,3,4 metabolic activity,5,6 and permeability of postcapillary venules.7 In 1976, Knight5 published an influential paper that introduced the so-called secondary injury model. Knight suggested that cryotherapy works by decreasing the metabolic activity of uninjured tissue, which, without cooling, would die or be secondarily injured by hypoxia resulting from compromised vasculature at the initial injury site.5 Subsequent research has shown that cold decreases blood flow and bone metabolism. Ho et al6 used triple-phase technetium bone scans to examine the effects of an ice wrap applied to a nontraumatized knee. Cooling with ice resulted in reduced (by 38.4%) arterial blood flow to the knee. The authors observed a 19.3% decrease in bone uptake of technetium and interpreted it to mean that cold reduced bone metabolism. Merrick et al21 reported that 5 hours of continuous cryotherapy inhibited secondary injury after crush injury to the skeletal muscle of rats. However, we are unaware of any controlled studies indicating that reducing secondary injury or metabolic activity significantly affects acute edema formation.
In contrast, numerous authors have suggested that acute edema results primarily from increased permeability of postcapillary venules to plasma macromolecules.2,22–24 Rippe and Grega7 showed that cooling (10°C–15°C [50°F–59°F]) decreased the histamine-induced permeability of vascular beds in rats. They suggested that cold diminished the development of gaps in venule walls, which greatly reduced protein and fluid loss to interstitium. They also suggested that cold could be as important as decreased blood flow in controlling edema formation. Clearly, more research on the physiologic mechanisms of cryotherapy and electric stimulation is warranted. Our research does not elucidate why CWI curbs acute edema formation, but the work of Rippe and Grega7 suggests that CWI directly affects permeability of postcapillary venules.
The physiologic mechanisms of how certain forms of high-voltage electric stimulation curb edema formation are not fully understood. Results of this study and others from our laboratory have repeatedly shown that CHVPC at 120 PPS and 90% of visible motor threshold is effective in curbing edema formation in rats and frogs.10–12 Reed,13 who introduced fluorescence-labeled dextran into hamsters to measure plasma protein leakage from cheek-pouch microvessels, provided the first compelling evidence that high-voltage pulsed current (HVPC) might be capable of minimizing edema formation. Application of histamine increased the number of leaks of labeled dextran from microvessels seen by fluorescence microscopy, but simultaneous application of 120-PPS HVPC at amplitudes of 30 or 50 V significantly reduced the number of leaks. Reed concluded that HVPC, as he applied it, reduced vascular permeability to plasma macromolecules. Subsequent work from our laboratory, tracking labeled dextran in microvessels, has also demonstrated that HVPC reduces permeability of microvessels.14,25
A review of nonhuman animal studies designed to determine efficacy of cold therapy in management of edema produced conflicting results. Farry et al26 reported that traumatized radiocarpal ligaments of domestic pigs, cooled by ice, showed less histologic evidence of inflammation but also showed increased swelling; also, application of crushed ice caused swelling in nontraumatized limbs. McMaster and Liddle27 examined the effects of immersion baths (20°C and 30°C [68°F and 86°F]) on edema in rabbit limbs after crush injury. A 1-hour treatment at 30°C resulted in the least edema when measured 24 hours postinjury. However, limbs of control animals, which received crush injuries but no cryotherapy, had less swelling than limbs treated at either 20°C or 30°C.
Matsen et al18 used New Zealand white rabbits as subjects to determine the effects of cold therapy on mechanically induced midshaft tibial fractures. They assessed prefracture volumes using a water-displacement system, and they treated limbs for 6 or 24 hours, with water ranging in temperature from 5°C to 25°C (41°F–77°F). Limbs were measured immediately after treatment, 6 hours after treatment, and then daily for 4 consecutive days. Limbs that were cooled to between 5°C and 15°C (41°F and 59°F) were significantly more swollen than control limbs (32°C [89.6°F]) 6 hours after treatment. Limbs cooled to 20°C to 25°C (68°F–77°F) were not different from control limbs at any time. Matsen et al18 concluded that there was no benefit to cooling at temperatures above 15°C and a detrimental effect with temperatures below 15°C. In 1973, Jezdinsky et al28 examined the effects of cold and heat on traumatized hind paws of rats and measured edema with a slide gauge rather than by volume displacement. Cooling limbs at 12.3°C (54.14°F) for 2 to 10 hours did not influence edema formation. However, after cold was removed, significant swelling occurred. Increases in edema formation after exposure to cold were reported in all 4 studies using nonhuman animals18,26–28; nevertheless, treatment times and temperatures were extreme relative to common clinical practice.
Research using nonhuman animal models to determine the efficacy of electric stimulation to control edema formation is conflicting. Results from this study and others from our laboratory have established that CHVPC at 120 PPS and 90% of visible motor threshold curbs edema formation, although animals (frogs and rats) remained in dependent position throughout experimentation.10–12,16,20
Mohr et al,29 who were the first to conduct controlled experiments to determine the effects of HVPC on existing edema, reported no treatment effect. They traumatized 1 hind limb of a series of rats, and after 24, 48, and 72 hours, they treated half the animals with HVPC at 80 PPS and 40 V for 20 minutes. Animals were unrestrained except during treatment, so muscle activity might have influenced limb volumes. Volumes of treated limbs did not differ significantly from untreated limbs. Similarly, Cosgrove et al30 compared 1-hour exposure to monophasic pulsed current and symmetric biphasic pulsed current on traumatized rat paws at 24, 48, 72, and 96 hours posttrauma. One hour of treatment over the 3-day period failed to demonstrate significant edema reduction in either treatment group. Animals were again unrestrained between treatments, and the authors stated that muscle activity probably facilitated fluid uptake, which could influence limb volumes.
Apparently, the trial reported here is the first randomized controlled study completed to determine the efficacy of combining cryotherapy and electrotherapy to curb edema formation. Rats were exposed to simultaneous application of CWI at 12.8°C (55°F) and CHVPC at 120 PPS and 90% of visible motor threshold. We hypothesized that if different treatment modalities affect different root causes of edema formation, then simultaneous application might produce a significantly greater reduction in edema formation. However, this did not occur in our experiment. We were surprised by this specific outcome but not by our observation that combining CWI and CHVPC curbs edema.
A review of human studies designed to determine the efficacy of cryotherapy and electric stimulation suggests that cold is interpreted to be effective in controlling edema, whereas electric stimulation is not. However, the few relevant studies suffer from poor experimental design or lack of proper controls. Basur et al31 treated ankle injuries with crepe bandaging or cryotherapy for 48 hours followed by crepe bandaging. The number of patients who were pain free and without restricted ankle movement was greater at 2 days and 7 days postinjury in the group treated with cryotherapy than in the group that had not received cryotherapy. However, it was not clear whether the cold gel packs used to transmit cold restricted movement more than in the bandage-only group. It is possible that the difference between the 2 groups was more a reflection of mobility than effectiveness of cold treatment. Sloan et al32 treated patients within 24 hours of injury with a single 30-minute application of a cold pack and elevation and sustained treatment with nonsteroidal anti-inflammatory medication. Seven days later, the patients were examined and compared with a group of patients who had not received cold or elevation but who had been treated with nonsteroidal anti-inflammatory drugs. Not surprisingly, the authors concluded that a single cold treatment had little effect.
Two other studies often cited in support of the efficacy of cryotherapy lacked proper control groups. Hocutt et al33 compared cryotherapy with heat therapy in patients with ankle sprains. Those receiving cold treatment and adhesive bandages within 36 hours of injury recovered more quickly than those receiving heat and elastic bandages. However, it is unclear whether the patients treated with cold recovered more quickly or if the recovery of those treated with heat was inhibited. Additionally, it is impossible to know if those treated with cold recovered more quickly than if they had not been treated with cold, because a control group without treatment was not included in this study.
Coté et al34 examined the effects of cold, heat, and contrast baths on subacute ankle injury. All 3 treatments increased edema, although cold caused less increase than the other two. However, cold had virtually no effect on existing edema, because volumes were essentially the same before and after treatments. Conversely, volumes measured after treatment with heat or contrast baths increased. None of the treatments as applied by Coté et al reduced existing edema. Michlovitz et al35 examined the effects of ice plus HVPC on acute grade 1 and 2 ankle sprains, concluding that volumes were not significantly different. Treatment began between 1 and 28 hours after injury. It seems that significant edema had already formed before treatments were applied. In addition, changes in volumes after treatments were frequently less than the measurement error determined during reliability testing.
For our immersion technique, we used warmer water than typically used by clinicians. We did so because review of the few controlled studies using ice water suggested that exposure to near-freezing water, even for short periods, causes edema formation, whereas exposure to slightly warmer temperatures may be therapeutic. In addition, earlier work from our group8 demonstrated that water between 12.8°C and 15°C (55°F and 60°F) curbed edema formation in traumatized rat limbs. Our current study confirms that somewhat warmer water than typically applied clinically does indeed curb edema formation. Similarly, our study reconfirms10–12 that CHVPC, as applied here, has a demonstrable treatment effect. A new finding in this study was that the combination of CWI and CHVPC has a treatment effect, but it is statistically indistinguishable from that produced by individual application of CWI or CHVPC.
We applied repeated 30-minute treatments interposed with 30-minute rest periods over a 4-hour period. This regime far exceeded typical clinical practice. We chose our protocol in this study to mimic that in our previous works. Moreover, we conducted 4-hour trials because we determined (in unpublished work) that the trauma we induced to the feet of anesthetized rats caused edema without causing bleeding. Bleeding causes limb volumes to increase but obviously not via edema. We observed that this level of trauma caused edema formation that continued for about 4 hours before beginning to abate. We were less interested in determining whether any of our putative therapies accelerated the natural diminution of edema, so we applied them only when we knew edema was still forming. We applied 30-minute treatments to simulate what we believed to be standard clinical practice. Applying several 30-minute treatments allowed us to determine the short-term effects of a single treatment and the cumulative effects of multiple treatments.
None of the treatments applied in this study completely precluded edema formation (see Figure 2). Indeed, all treated limbs swelled, albeit statistically less than untreated limbs. Less edema occurred when treatments were applied, and more occurred during rest periods. This “stepped curve” was most obvious for CHVPC but was discernible for CWI and the combination of CWI and CHVPC. We speculate that the more obvious stepped curve of CHVPC resulted from the fully “on” versus fully “off” nature of that modality. The “steps” were less obvious when we applied CWI only or CWI and CHVPC simultaneously, presumably because limbs did not immediately cool or warm to body temperature when withdrawn from cool water and placed in water that was warmer but still cooler than thermoneutral water. In other words, tissues took some time to chill when immersed in cool water (12.8°C), and similarly, took some time to warm when removed from it. Hence, tissues were not instantly exposed to or removed from seemingly therapeutic environments as they were when exposed to CHVPC.
The application of only a single 30-minute treatment of CWI or CHVPC or the combination of CWI and CHVPC anytime other than very early during a 4-hour trial (when edema was forming) almost certainly would not have resulted in a discernible treatment effect at the end of a trial. If rats model human responses to the putative therapies applied here, then we suggest that a single 30-minute application of any therapy would have virtually no clinical effect. The statistical treatment effects that we observed accumulated over repeated applications. Edema increased faster during rest periods between treatments. This pattern suggests that continuously applying any of the treatments used here during the time edema is still forming would result in a greater curbing of edema formation than we observed in these trials. We are now conducting a study to test this hypothesis.
Our trial design did not address the mechanisms by which CWI, CHVPC, or the combination might curb edema formation. However, we had evidence from previous work from our group14,24 and others13 that HVPC curbs edema formation by decreasing the permeability of microvessels. Rippe and Grega7 suggested a similar mechanism for CWI, but Knight1,5 has long advocated that reducing metabolic activity in periwound areas, via application of cold, reduces secondary injury and hence, reduces edema formation. We thought it worthwhile to simultaneously apply CWI and CHVPC to see first if the common, but unsubstantiated, clinical practice of combining these 2 modalities would show a treatment effect under controlled conditions. If it did, and the modalities did curb edema formation via different mechanisms, then an additive effect might be in evidence. We observed a treatment effect but it was not statistically distinguishable from the application of CWI or CHVPC alone. In other words, there was no additive effect. This implies to us that both CWI and CHVPC might curb edema by affecting the same mechanism. Our bias at the moment, based on our own work14,24 and that of Reed13 and Rippe and Grega,7 is that both modalities affect the permeability of microvessels.
ACKNOWLEDGMENTS
We thank Carl Mattacola, PhD, ATC, of the University of Kentucky for reviewing the paper and statistical consultation. This project was funded by a grant from the New York State Athletic Trainers' Association and a Canisius College Excellence Earning Grant.
REFERENCES
- 1.Knight KL. Cryotherapy in Sports Injury Management. Champaign, IL: Human Kinetics; 1995. [Google Scholar]
- 2.Wilkerson GB, Horn-Kingery HM. Treatment of the inversion ankle sprain: comparison of different modes of compression and cryotherapy. J Orthop Sports Phys Ther. 1993;17:240–246. doi: 10.2519/jospt.1993.17.5.240. [DOI] [PubMed] [Google Scholar]
- 3.Knight KL, Londeree BR. Comparison of blood flow in the ankle of uninjured subjects during therapeutic applications of heat, cold, and exercise. Med Sci Sports Exerc. 1980;12:76–80. doi: 10.1249/00005768-198021000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Weston M, Taber C, Casagranda L, Cornwall M. Changes in local blood volume during cold gel pack application to traumatized ankles. J Orthop Sports Phys Ther. 1994;19:197–199. doi: 10.2519/jospt.1994.19.4.197. [DOI] [PubMed] [Google Scholar]
- 5.Knight K. The effects of hypothermia on inflammation and swelling. J Athl Train. 1976;11:7–10. [Google Scholar]
- 6.Ho SS, Coel MN, Kagawa R, Richardson AB. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537–540. doi: 10.1177/036354659402200417. [DOI] [PubMed] [Google Scholar]
- 7.Rippe B, Grega GJ. Effects of isoprenaline and cooling on histamine induced changes of capillary permeability in the rat hindquarter vascular bed. Acta Physiol Scand. 1978;103:252–262. doi: 10.1111/j.1748-1716.1978.tb06212.x. [DOI] [PubMed] [Google Scholar]
- 8.Dolan MG, Thornton RM, Fish DR, Mendel FC. Effects of cold water immersion on edema formation after blunt injury to the hind limbs of rats. J Athl Train. 1997;32:233–237. [PMC free article] [PubMed] [Google Scholar]
- 9.Norwig JA. Injury management update: edema control and the acutely inverted ankle sprain. Athl Ther Today. 1997;2(1):40–41. [Google Scholar]
- 10.Bettany JA, Fish DR, Mendel FC. Influence of high voltage pulsed direct current on edema formation following impact injury. Phys Ther. 1990;70:219–224. doi: 10.1093/ptj/70.4.219. [DOI] [PubMed] [Google Scholar]
- 11.Bettany JA, Fish DR, Mendel FC. High-voltage pulsed direct current: effect on edema formation after hyperflexion injury. Arch Phys Med Rehabil. 1990;71:677–681. [PubMed] [Google Scholar]
- 12.Mendel FC, Wylegala JA, Fish DR. Influence of high voltage pulsed current on edema formation following impact injury in rats. Phys Ther. 1992;72:668–673. doi: 10.1093/ptj/72.9.668. [DOI] [PubMed] [Google Scholar]
- 13.Reed BV. Effect of high voltage pulsed electrical stimulation on microvascular permeability to plasma proteins: a possible mechanism in minimizing edema. Phys Ther. 1988;68:491–495. doi: 10.1093/ptj/68.4.491. [DOI] [PubMed] [Google Scholar]
- 14.Taylor K, Mendel FC, Fish DR, Hard R, Burton HW. Effect of high-voltage pulsed current and alternating current on macromolecular leakage in hamster cheek pouch microcirculation. Phys Ther. 1997;77:1729–1740. doi: 10.1093/ptj/77.12.1729. [DOI] [PubMed] [Google Scholar]
- 15.Karnes JL, Mendel FC, Fish DR, Burton HW. High-voltage pulsed current: its influence on diameters of histamine-dilated arterioles in hamster cheek pouches. Arch Phys Med Rehabil. 1995;76:381–386. doi: 10.1016/s0003-9993(95)80665-2. [DOI] [PubMed] [Google Scholar]
- 16.Thornton RM, Mendel FC, Fish DR. Effects of electrical stimulation on edema formation in different strains of rats. Phys Ther. 1998;78:386–394. doi: 10.1093/ptj/78.4.386. [DOI] [PubMed] [Google Scholar]
- 17.Dolan MG, Mendel FC, Teprovich JM, Marvar PJ, Bibi KW. Effects of dependent positioning and cold water immersions on non-traumatized ankle volumes [abstract] J Athl Train. 1999;34(suppl):S-17. [Google Scholar]
- 18.Matsen FA, III, Questad K, Matsen AL. The effect of local cooling on postfracture swelling: a controlled study. Clin Orthop. 1975;109:201–206. doi: 10.1097/00003086-197506000-00029. [DOI] [PubMed] [Google Scholar]
- 19.Green CJ. Animal Anesthesia. London, England: Laboratory Animals Ltd; 1982. General principles. [Google Scholar]
- 20.Mendel FC, Fish DR. New perspectives in edema control via electrical stimulation. J Athl Train. 1993;28:63–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31:1516–1521. doi: 10.1097/00005768-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Grega GJ, Adamski SW. The role of venular endothelial cells in the regulation of macromolecular permeability. Microcirc Endothelium Lymphatics. 1988;4:143–167. [PubMed] [Google Scholar]
- 23.Grega GJ, Adamski SW, Dobbins DE. Physiological and pharmacological evidence for the regulation of permeability. Fed Proc. 1986;45:96–100. [PubMed] [Google Scholar]
- 24.Denegar CR. Therapeutic Modalities for Athletic Injuries. Champaign, IL: Human Kinetics; 2000. [Google Scholar]
- 25.Karnes JL, Mendel FC, Fish DR, Burton HW. High-voltage pulsed current: its influence on diameters of histamine-dilated arterioles in hamster cheek pouches. Arch Phys Med Rehabil. 1995;76:381–386. doi: 10.1016/s0003-9993(95)80665-2. [DOI] [PubMed] [Google Scholar]
- 26.Farry PJ, Prentice NG, Hunter AC, Wakelin CA. Ice treatment of injured ligaments: an experimental model. N Z Med J. 1980;91:12–14. [PubMed] [Google Scholar]
- 27.McMaster WC, Liddle S. Cryotherapy influence on posttraumatic limb edema. Clin Orthop. 1980;150:283–287. [PubMed] [Google Scholar]
- 28.Jezdinsky J, Marek J, Ochonsky P. Effects of local cold and heat therapy on traumatic oedema of the rat hind paw, I: effects of cooling on the course of traumatic oedema. Acta Univ Pakacki Olomuc Fac Medicae. 1973;66:185–201. [Google Scholar]
- 29.Mohr TM, Akers TK, Landry RG. Effect of high voltage stimulation on edema reduction in the rat hind limb. Phys Ther. 1987;67:1703–1707. doi: 10.1093/ptj/67.11.1703. [DOI] [PubMed] [Google Scholar]
- 30.Cosgrove KA, Alon G, Bell SF, et al. The electrical effect of two commonly used clinical stimulators on traumatic edema in rats. Phys Ther. 1992;72:227–233. doi: 10.1093/ptj/72.3.227. [DOI] [PubMed] [Google Scholar]
- 31.Basur RL, Shephard E, Mouzas GL. A cooling method in the treatment of ankle sprains. Practitioner. 1976;216:708–711. [PubMed] [Google Scholar]
- 32.Sloan JP, Hain R, Pownall R. Clinical benefits of early cold therapy in accident and emergency following ankle sprain. Arch Emerg Med. 1989;6:1–6. doi: 10.1136/emj.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hocutt JE, Jr, Jaffe R, Rylander CR, Beebe JK. Cryotherapy in ankle sprains. Am J Sports Med. 1982;10:316–319. doi: 10.1177/036354658201000512. [DOI] [PubMed] [Google Scholar]
- 34.Coté DJ, Prentice WE, Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther. 1988;68:1072–1076. doi: 10.1093/ptj/68.7.1072. [DOI] [PubMed] [Google Scholar]
- 35.Michlovitz S, Smith W, Watkins M. Ice and high voltage pulsed stimulation in treatment of acute lateral ankle sprains. J Orthop Sports Phys Ther. 1988;9:301–304. doi: 10.2519/jospt.1988.9.9.301. [DOI] [PubMed] [Google Scholar]