Abstract
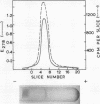
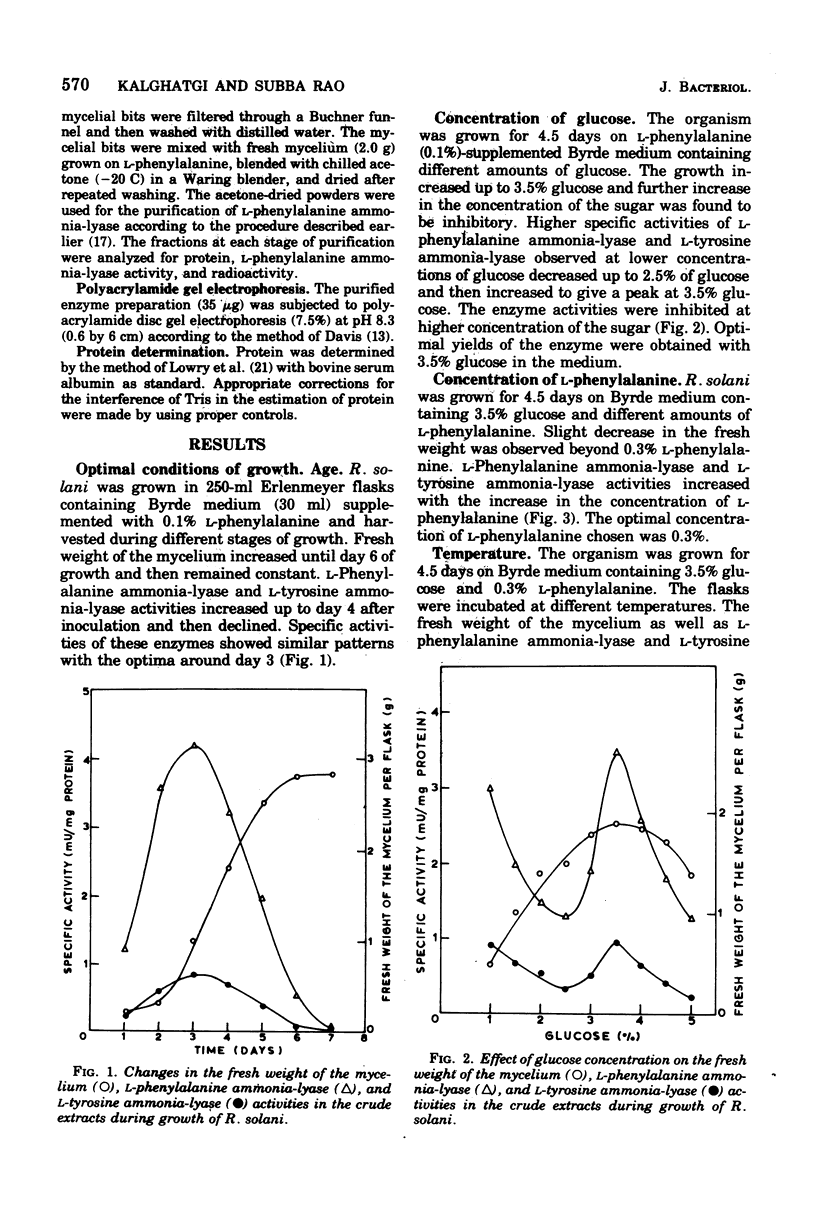
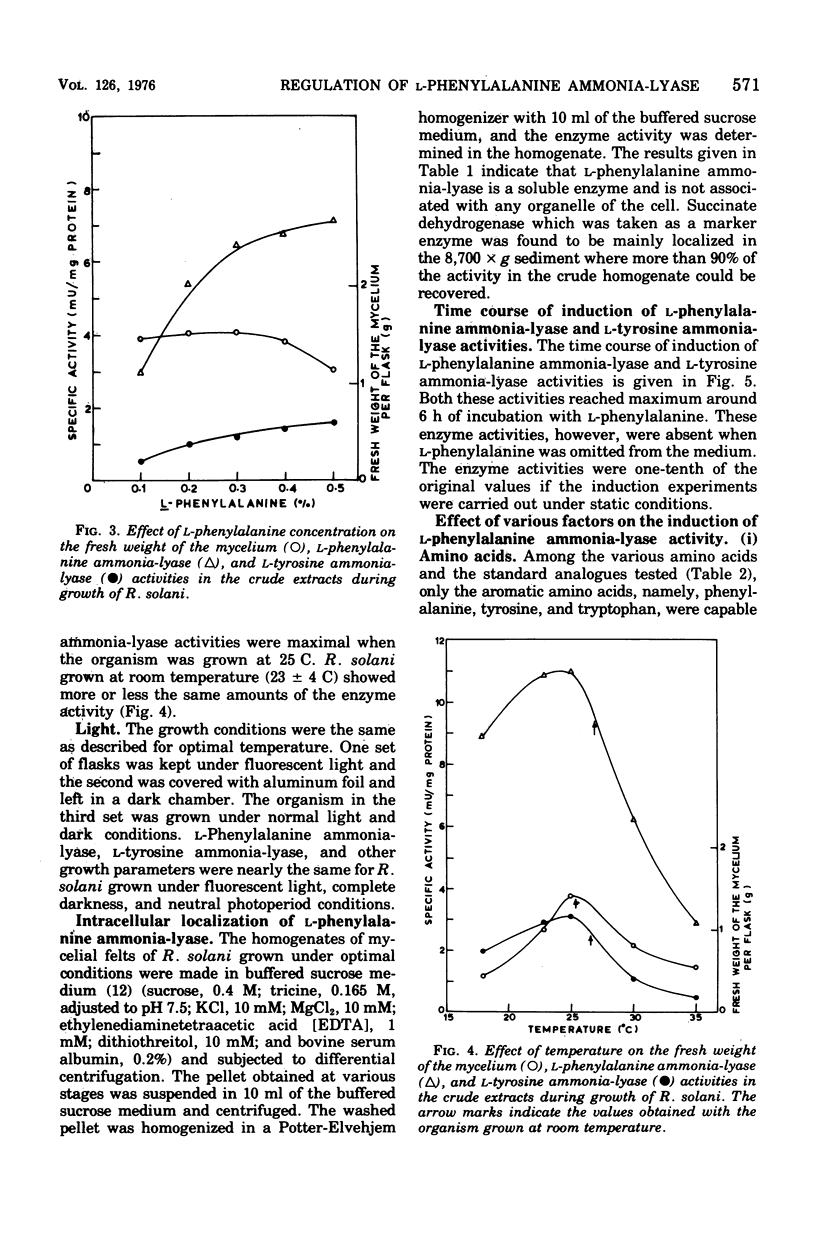
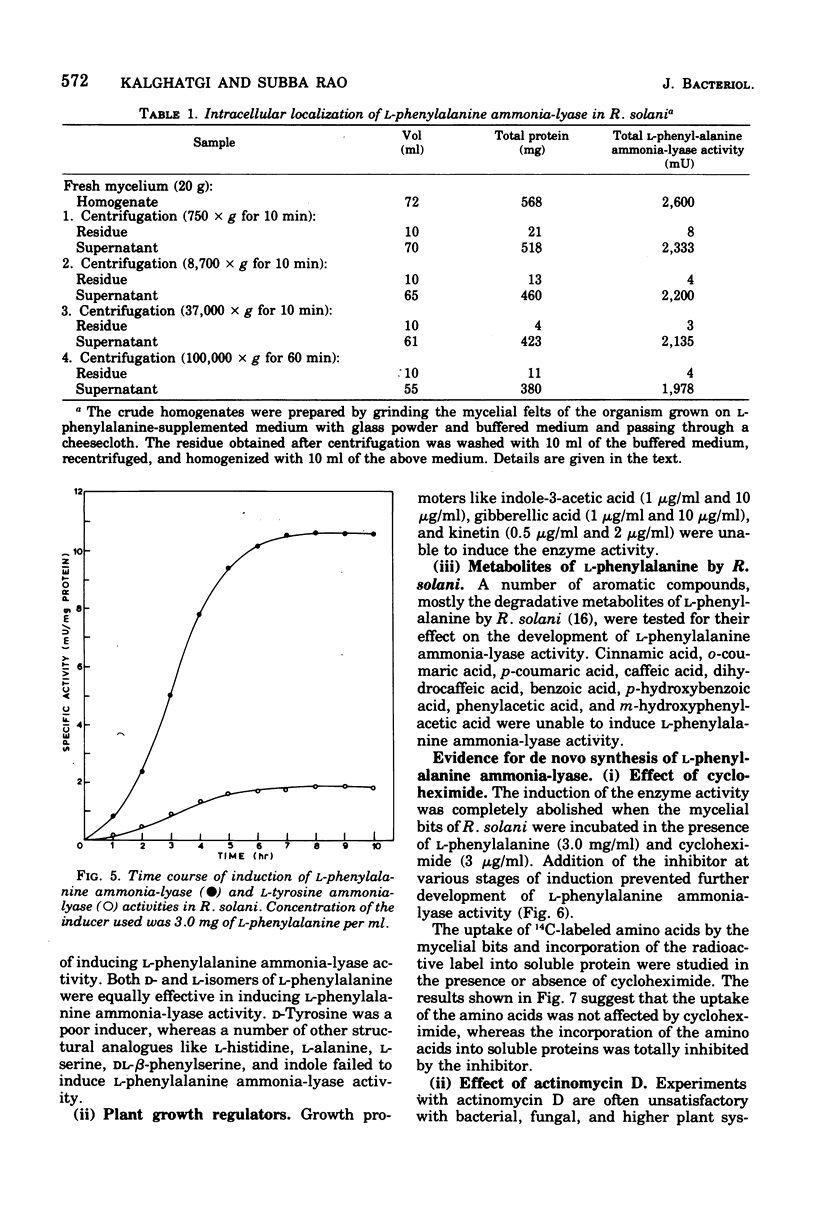
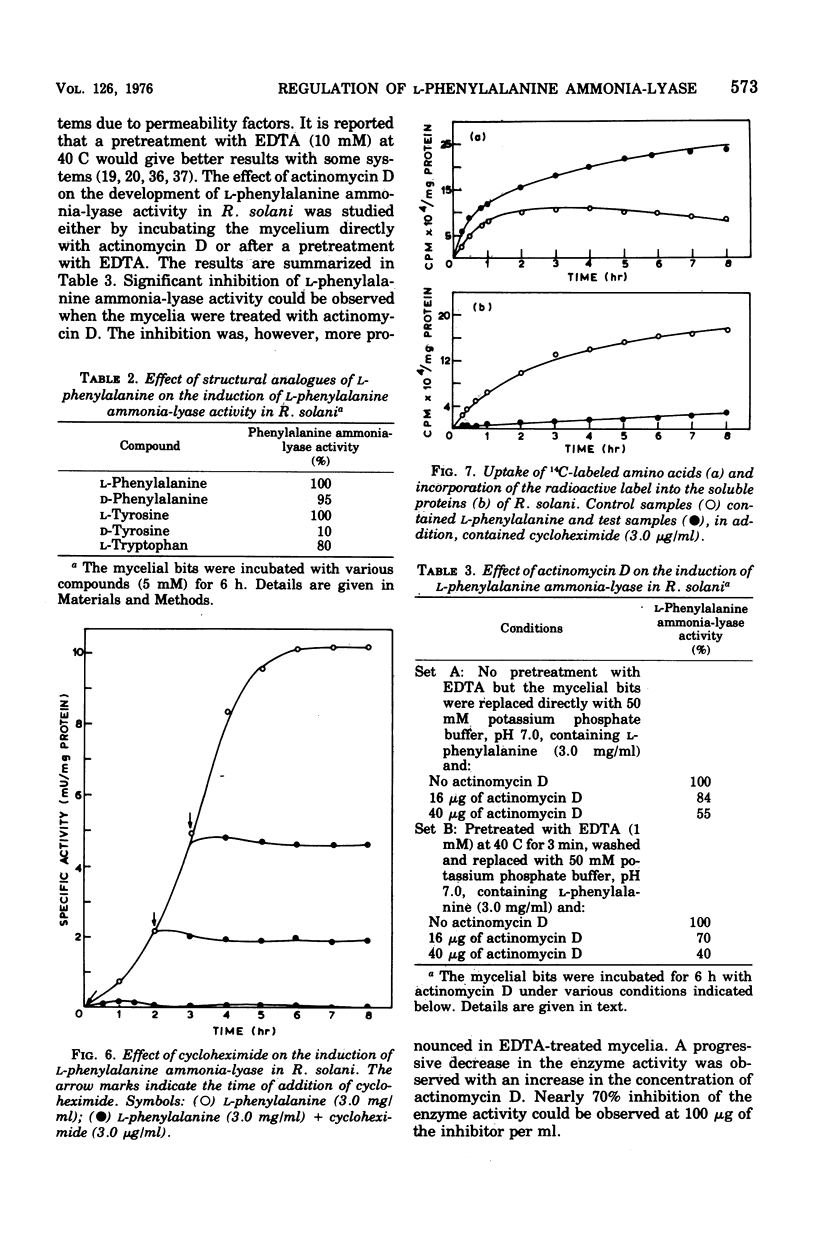
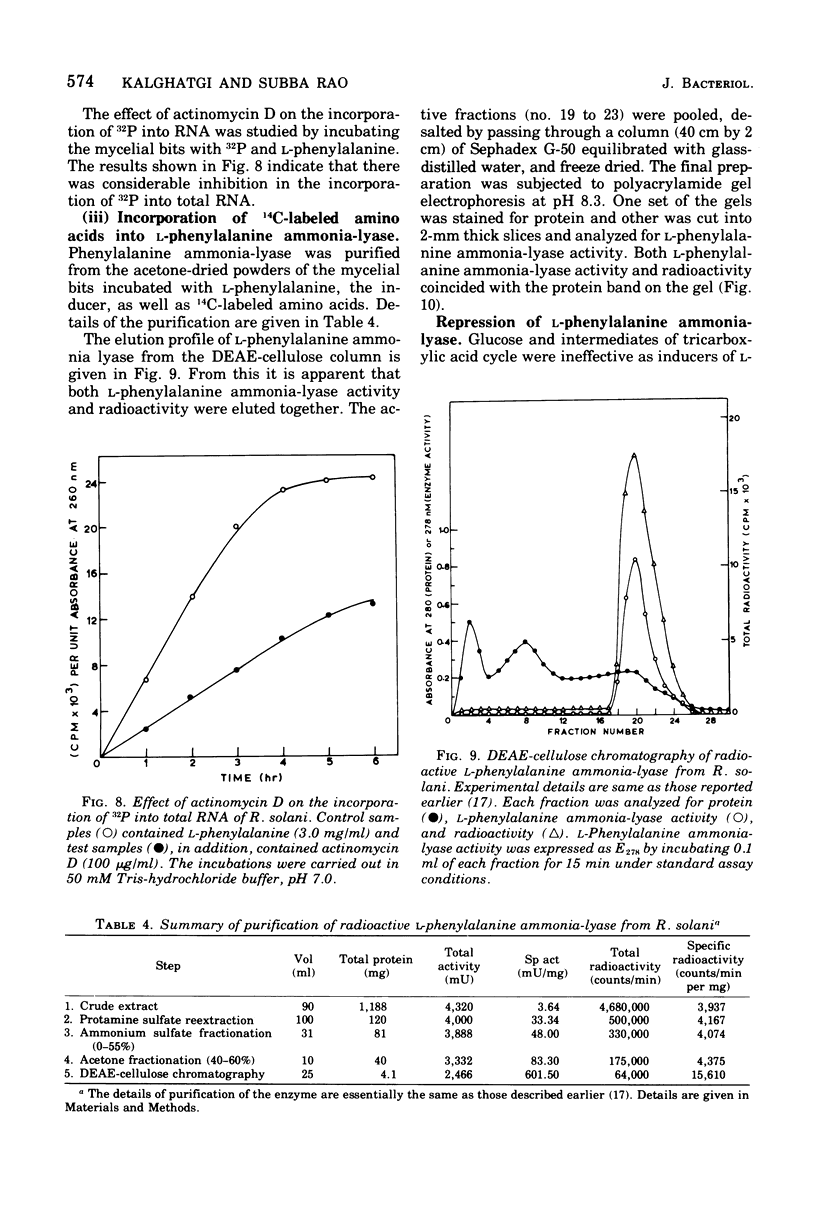
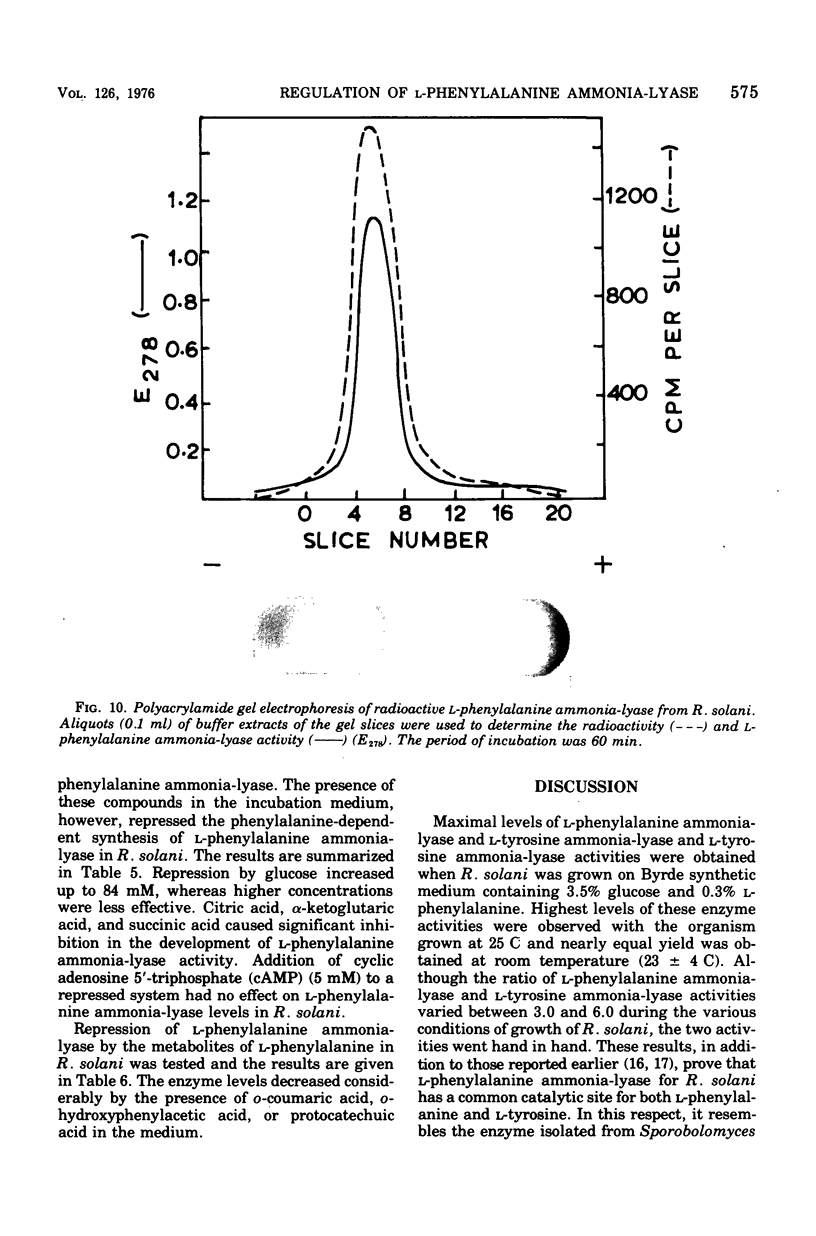
Maximal levels of L-henylalanine ammonia-lyase activity were observed when the mycelial felts of Rhizoctonia solani were grown for 4.5 days on Byrde synthetic medium containing 3.5% glucose and 0.3% L-phenylalanine, Differential centrifugation studies have indicated that the enzyme is localized in the soluble fraction. The time course of induction of L-phenylalanine ammonia-lyase activity by L-phenylalanine showed a lag period of 1 to 1.5 h and reached a maximum around 4 to 6 h after the addition of the inducer to the medium. L-Phenylalanine, L-tyrosine, and L-tryptophan were nearly equally efficient inducers of the enzyme. D-Phenylalanine was as efficient as the L-isomer, whereas D-tyrosine was a poor inducer. Light, gibberellic acid, indole 3-acetic acid, and kinetin had no effect on the induction of L-phenylalanine ammonia-lyase activity. Cycloheximide did not inhibit the uptake of amino acids by the mycelia but completely blocked the incorporation of radioactive amino acids into soluble proteins and the development of L-phenylalanine ammonia-lyase activity. Actinomycin D inhibited both the incorporation of 32P into ribonucleic acid and the enzyme activity. Conclusive evidence for de novo synthesis of L-phenylalanine ammonia-lyase was obtained by the incorporation of radioactive amino acids into the enzyme. Electrophoretic analysis of the purified preparation showed a single protein band that coincided with radioactivity and L-phenylalanine ammonia-lyase activity. Glucose and intermediates of the tricarboxylic acid cycle, like citric acid, alpha-ketoglutaric acid, and succinic acid, and the metabolites of L-phenylalanine, like o-coumaric acid, o-hydroxyphenylacetic acid, and protocatechuic acid, significantly repressed L-phenylalanine ammonia-lyase activity. The observed repression was not relieved by cyclic adenosine 5'-triphosphate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alibert G., Ranjeva R., Boudet A. Recherches sur les enzymes catalysant la formation des acides phénoliques chez Quercus pedunculata (Ehrh.). II. Localisation intracellulaire de la phenylalanine ammoniaque-lyase, de la cinnamate 4-hydroxylase, et de la "benzoate synthase". Biochim Biophys Acta. 1972 Sep 15;279(2):282–289. [PubMed] [Google Scholar]
- Austin D. J., Clarke D. D. Production of coumarins by Phytophthora infestans. Nature. 1966 Jun 11;210(5041):1165–1166. doi: 10.1038/2101165a0. [DOI] [PubMed] [Google Scholar]
- BENNETT L. L., Jr, SMITHERS D., WARD C. T. INHIBITION OF DNA SYNTHESIS IN MAMMALIAN CELLS BY ACTIDIONE. Biochim Biophys Acta. 1964 May 18;87:60–69. doi: 10.1016/0926-6550(64)90047-7. [DOI] [PubMed] [Google Scholar]
- BYRDE R. J., HARRIS J. F., WOODCOCK D. Fungal detoxication, the metabolism of -(2-naphthyloxy)-n-alkylcarboxylic acids by Aspergillus niger. Biochem J. 1956 Sep;64(1):154–160. doi: 10.1042/bj0640154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanson G. S., Desaty D., Emes A. V., Vining L. C. Biosynthesis of cinnamamide and detection of phenylalanine ammonia-lyase in Streptomyces verticillatus. Can J Microbiol. 1970 Mar;16(3):147–151. doi: 10.1139/m70-026. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Schröder J. Light-induced changes of enzyme activities in parsley cell suspension cultures. Increased rate of synthesis of phenylalanine ammonia-lyase. Arch Biochem Biophys. 1975 Jan;166(1):47–53. doi: 10.1016/0003-9861(75)90363-x. [DOI] [PubMed] [Google Scholar]
- Havir E. A. l-Phenylalanine Ammonia-Lyase (Maize): Evidence for a Common Catalytic Site for l-Phenylalanine and l-Tyrosine. Plant Physiol. 1971 Aug;48(2):130–136. doi: 10.1104/pp.48.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalghatgi K. K., Nambudiri A. M., Bhat J. V., Subba Rao P. V. Degradation of L-penylalanine by Rhizoctonia solani. Indian J Biochem Biophys. 1974 Jun;11(2):116–118. [PubMed] [Google Scholar]
- Kalghatgi K. K., Subba Rao P. V. Microbial L-phenylalanine ammonia-lyase. Purification, subunit structure and kinetic properties of the enzyme from Rhizoctonia solani. Biochem J. 1975 Jul;149(1):65–72. doi: 10.1042/bj1490065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore K., Towers G. H. Degradation of aromatic amino acids by fungi. I. Fate of L-phenylalanine in Schizophyllum commune. Can J Biochem. 1967 Nov;45(11):1659–1665. doi: 10.1139/o67-196. [DOI] [PubMed] [Google Scholar]
- Rao P. V., Moore K., Towers G. H. Degradation of aromatic amino acids by fungi. II. Purification and properties of phenylalanine ammonia-lyase from Ustilago hordei. Can J Biochem. 1967 Dec;45(12):1863–1872. doi: 10.1139/o67-219. [DOI] [PubMed] [Google Scholar]
- Ross C. Influence of cycloheximide (Actidione) upon pyrimidine nucleotide metabolism and rna synthesis in cocklebur leaf discs. Biochim Biophys Acta. 1968 Aug 23;166(1):40–47. doi: 10.1016/0005-2787(68)90488-7. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Terry K., Matchett W. H. Temporal separation of transcription and translation in Neurospora. J Bacteriol. 1970 Aug;103(2):370–374. doi: 10.1128/jb.103.2.370-374.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urey J. C., Horowitz N. H. Effects of EDTA on tyrosinase and L-amino-acid oxidase induction in Neurospora crassa. Biochim Biophys Acta. 1967 Mar 15;132(2):300–309. doi: 10.1016/0005-2744(67)90149-0. [DOI] [PubMed] [Google Scholar]
- Zucker M. Induction of phenylalanine ammonia-lyase in Xanthium leaf disks. Photosynthetic requirement and effect of daylength. Plant Physiol. 1969 Jun;44(6):912–922. doi: 10.1104/pp.44.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]