Abstract
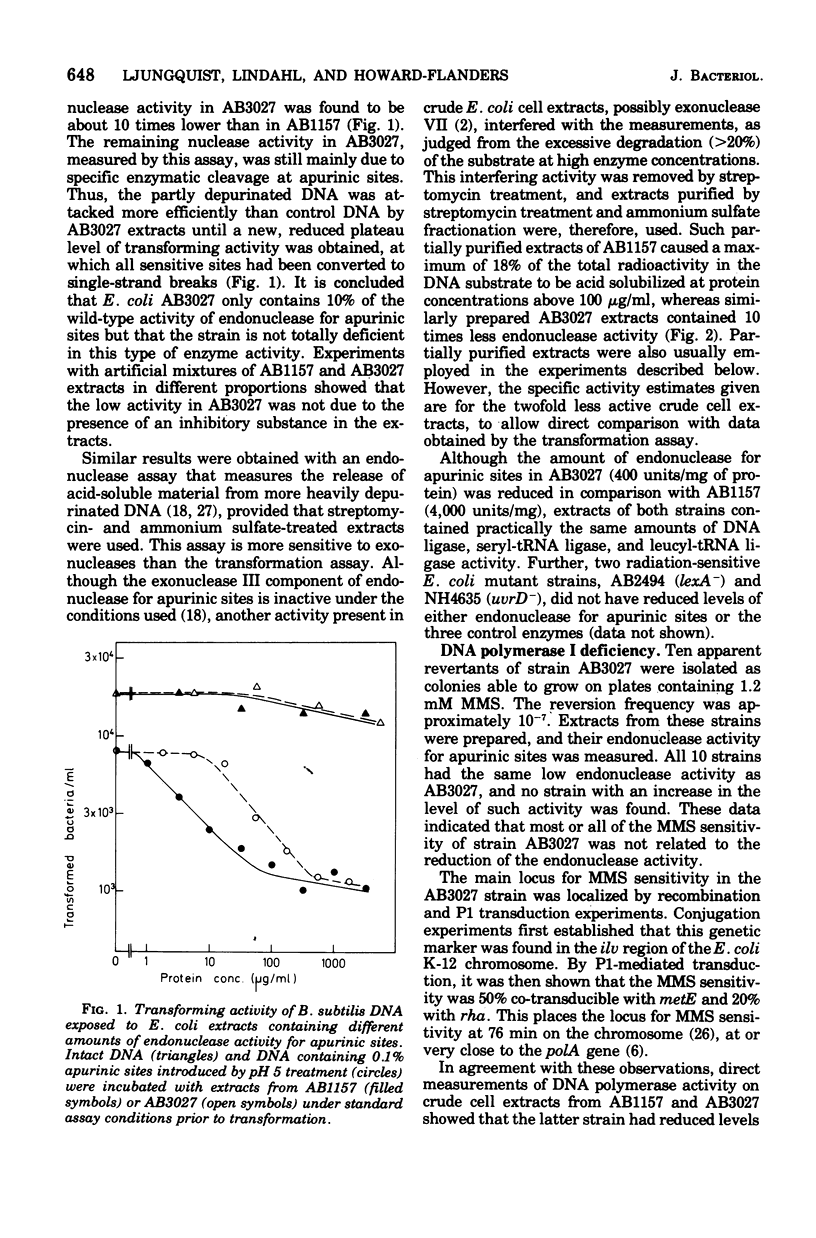
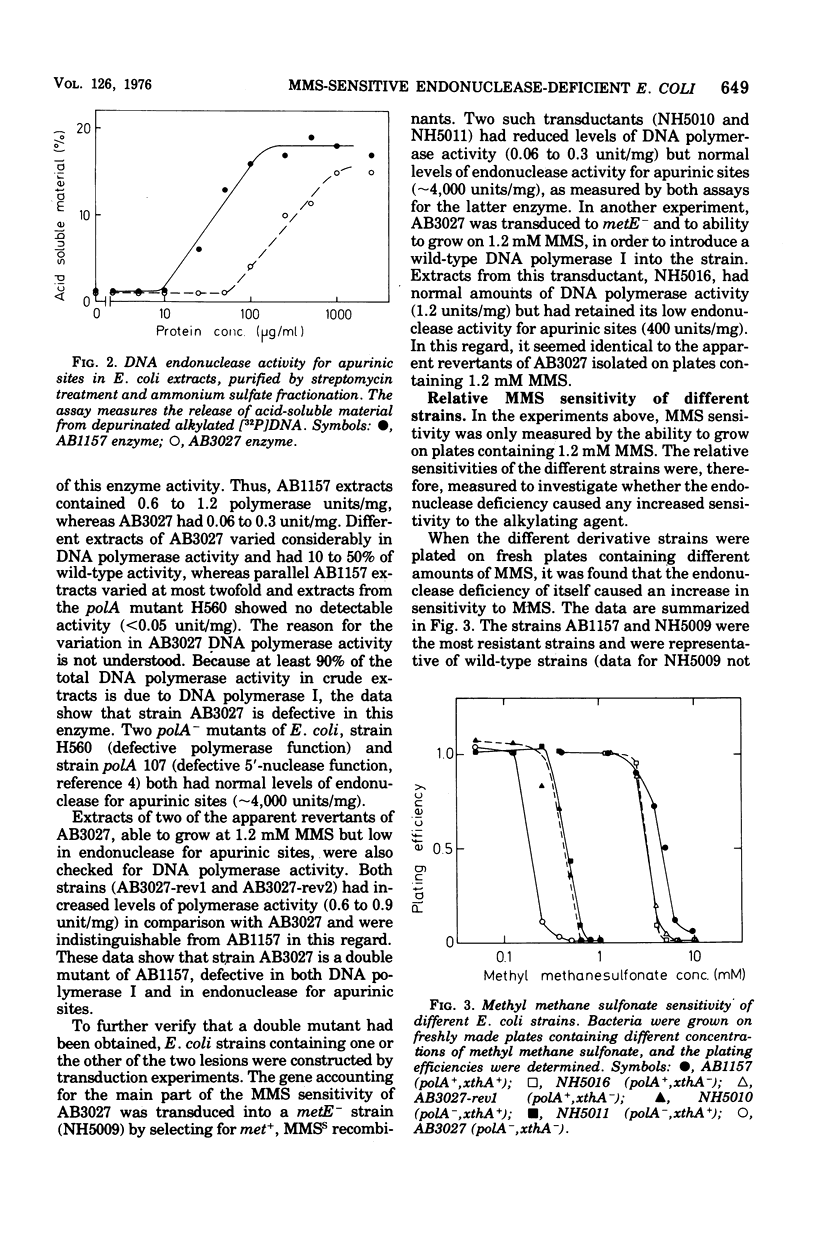
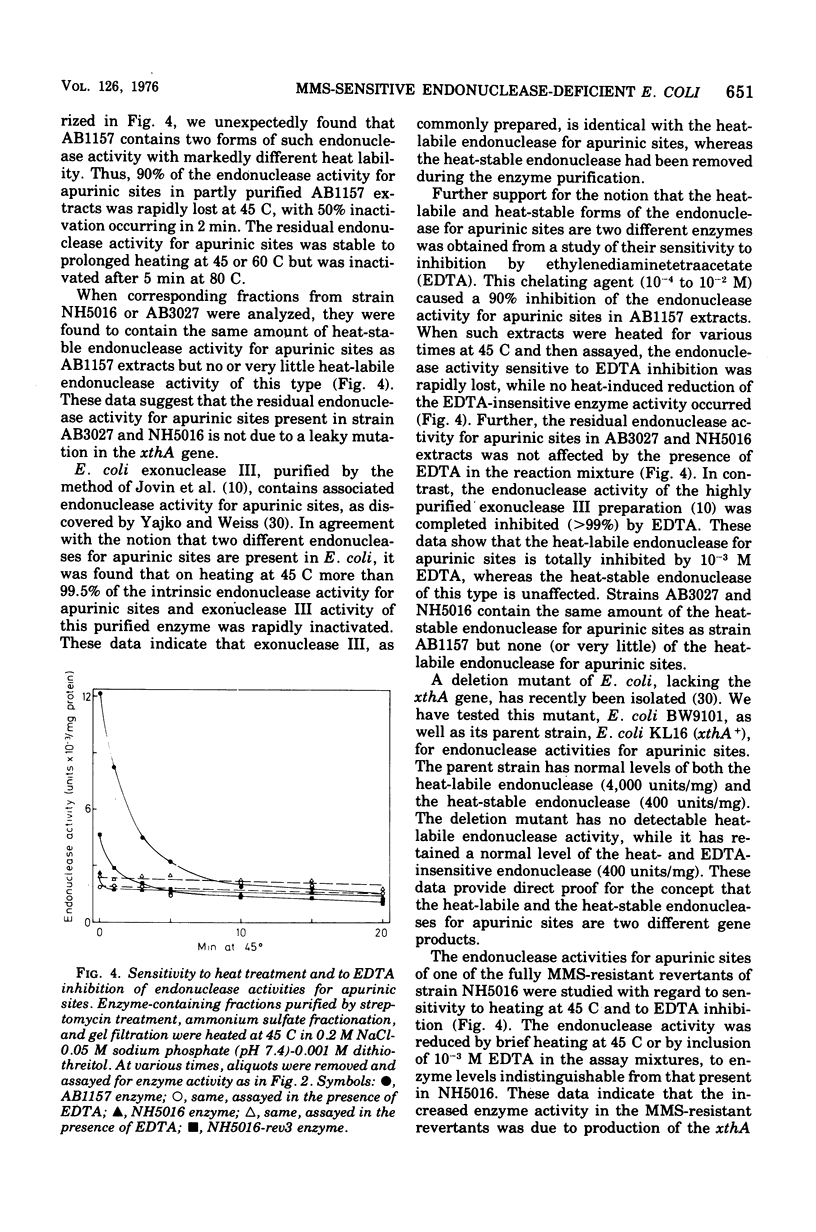
A methyl methane sulfonate (MMS)-sensitive mutant of Escherichia coli AB 1157 was obtained by N-methyl-N'-nitro-N-nitrosoguanidine treatment. The mutant strain, AB 3027, is defective both in endonuclease activity for apurinic sites in deoxyribonucleic acid (DNA) and in DNA polymerase I, as shown by direct enzyme assays. Derivative strains, which retained the deficiency in endonuclease activity for apurinic sties (approximately 10% of the wild-type enzyme level) but had normal DNA polymerase I activity, were obtained by P1-mediated transduction (strain NH5016) or by selection of revertants to decreased MMS sensitivity. These endonuclease-deficient strains are more MMS-sensitive than wild-type strains. Revertants of these deficients strains to normal MMS resistance were isolated. They had increased levels of the endonuclease activity but did not attain wild-type levels. The data suggest that endonuclease for apurinic sites is active in repair of lesions introduced in DNA as a consequence of MMS treatment. Two different endonucleases that specifically attack DNA containing apurinic sites arepresented in E coli K-12. A heat-labile activity, sensitive to inhibition by ethylenediaminetetraacetate, accounts for 90% of the total endonuclease activity for apurinic sties in crude cell extracts. The residual 10% is due to a more heat-resistant activity, refractory to ethylenediaminetetraacetate inhibition. The AB3027 and NH5016 strains have normal amounts of the latter endonuclease but no or very little of the former activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Friedberg E. C., Hadi S. M., Goldthwait D. A. Endonuclease II of Escherichia coli. II. Enzyme properties and studies on the degradation of alkylated and native deoxyribonucleic acid. J Biol Chem. 1969 Nov 10;244(21):5879–5889. [PubMed] [Google Scholar]
- Glickman B. W., van Sluis C. A., Heijneker H. L., Rörsch A. A mutant of Escherichia coli K12 deficient in the 5'-3' exonucleolytic activity of DNA polymerase I. I. General characterization. Mol Gen Genet. 1973 Jul 31;124(1):69–82. doi: 10.1007/BF00267166. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Goldthwait D. A. Endonuclease II of Escherichia coli. Degradation of partially depurinated deoxyribonucleic acid. Biochemistry. 1971 Dec 21;10(26):4986–4993. doi: 10.1021/bi00802a024. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Kirtikar D., Goldthwait D. A. Endonuclease II of Escherichia coli. Degradation of double- and single-stranded deoxyribonucleic acid. Biochemistry. 1973 Jul 3;12(14):2747–2754. doi: 10.1021/bi00738a030. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kirtikar D. M., Goldthwait D. A. The enzymatic release of O6-methylguanine and 3-methyladenine from DNA reacted with the carcinogen N-methyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1974 May;71(5):2022–2026. doi: 10.1073/pnas.71.5.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Fresco J. R. Renaturation of transfer ribonucleic acids through site binding of magnesium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):941–948. doi: 10.1073/pnas.55.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. New class of enzymes acting on damaged DNA. Nature. 1976 Jan 1;259(5538):64–66. doi: 10.1038/259064a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Ljungquist S., Andersson A., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. II. Further studies on the substrate specificity. J Biol Chem. 1974 Mar 10;249(5):1536–1540. [PubMed] [Google Scholar]
- Ljungquist S., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. I. Purification and general properties. J Biol Chem. 1974 Mar 10;249(5):1530–1535. [PubMed] [Google Scholar]
- Ljungquist S., Nyberg B., Lindahl T. Mammalian DNA endonuclease acting at apurinic sites: absence of associated exonuclease activity. FEBS Lett. 1975 Sep 15;57(2):169–171. doi: 10.1016/0014-5793(75)80708-3. [DOI] [PubMed] [Google Scholar]
- Margison G. P., O'Connor P. J. Biological implications of the instability of the N-glycosidic bone of 3-methyldeoxyadenosine in DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):349–356. doi: 10.1016/0005-2787(73)90021-x. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. doi: 10.1016/0022-2836(72)90215-x. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Preliminary mapping of mutations affecting exonuclease 3 in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):1086–1088. doi: 10.1128/jb.113.2.1086-1088.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- RICHARDSON C. C., SCHILDKRAUT C. L., APOSHIAN H. V., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XIV. FURTHER PURIFICATION AND PROPERTIES OF DEOXYRIBONUCLEIC ACID POLYMERASE OF ESCHERICHIA COLI. J Biol Chem. 1964 Jan;239:222–232. [PubMed] [Google Scholar]
- Ray U., Bartenstein L., Drake J. W. Inactivation of bacteriophage T4 by ethyl methanesulfonate: influence of host and viral genotypes. J Virol. 1972 Mar;9(3):440–447. doi: 10.1128/jvi.9.3.440-447.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B., Scudiero D., Henderson E. The nature of the alkylation lesion in mammalian cells. Basic Life Sci. 1975;5A:13–24. doi: 10.1007/978-1-4684-2895-7_2. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly W. G., Paquette Y., Thibodeau L. Nuclease for DNA apurinic sites may be involved in the maintenance of DNA in normal cells. Nat New Biol. 1973 Jul 18;244(133):67–69. doi: 10.1038/newbio244067a0. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Rassart E. Purification of Escherichia coli endonuclease specific for apurinic sites in DNA. J Biol Chem. 1975 Oct 25;250(20):8214–8219. [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Weiss B. Mutations simultaneously affecting endonuclease II and exonuclease III in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Feb;72(2):688–692. doi: 10.1073/pnas.72.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]



