Abstract
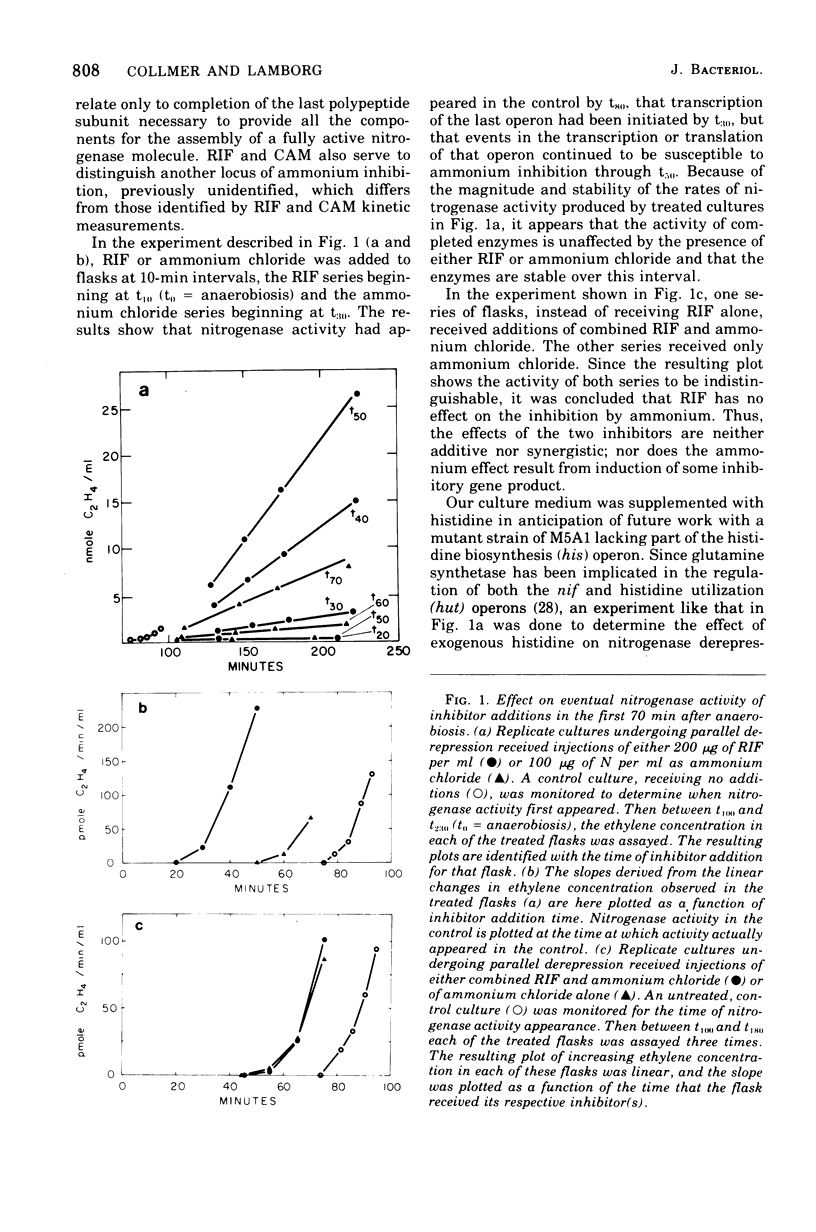
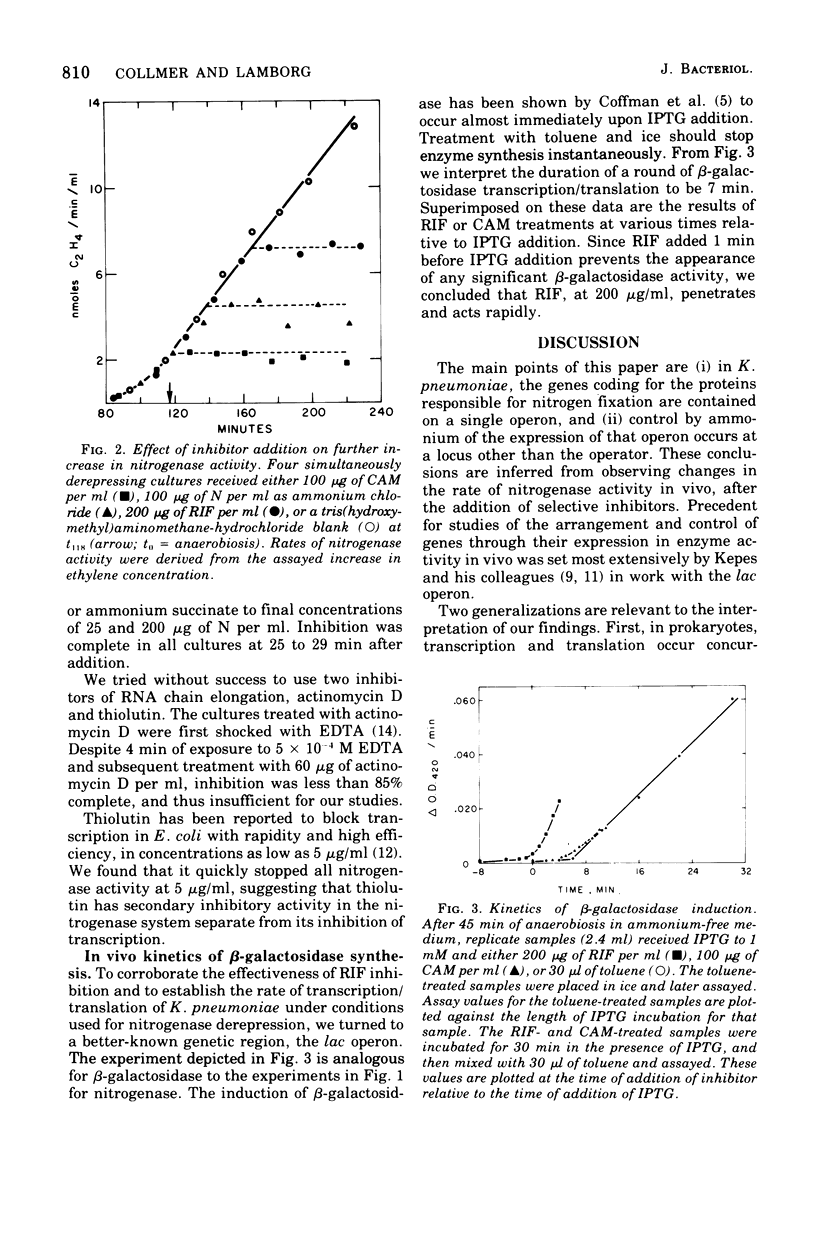
Events underlying derepression of the nitrogen fixation (nif) genes in Klebsiella pneumoniae M5A1 were analyzed in vivo by comparing the effects of selective inhibitors of transcription and translation on subsequent nitrogenase activity (rate of acetylene reduction). When batch cultures were induced for derepression, an 87-min lag separated ammonium ion/oxygen removal and the appearance of activity. To prevent eventual activity by adding inhibitors during this period, it was found necessary to add rifampin, ammonium ion, or chloramphenicol more than 50, 20, or 10 min, respectively, before activity appeared in a parallel control. When these inhibitors were added to cultures in which nitrogenase activity had already appeared, further increase in activity was not stopped by rifampin or ammonium ion until 45 or 20 min, respectively, after addition. Chloramphenicol stopped further increase in nitrogenase activity almost immediately. These data indicated a nif operon whose transcription/translation consumes 40 min. When the kinetics of β-galactosidase induction were analyzed under similar conditions, it was found that 7 min separated the initiation of transcription and the first completions of translation. Extrapolating from this, we find the nif operon to occupy 17 average gene lengths. It is argued that the nif structural genes in K. pneumoniae are contained on one operon, and further, that the disparity between the kinetics of inhibition by rifampin and those by ammonium ion suggests regulation at a locus other than the operator.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D., Kennell D. Metabolism of messenger RNA from the gal operon of Escherichia coli. J Mol Biol. 1974 Dec 15;90(3):581–599. doi: 10.1016/0022-2836(74)90236-8. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Bleyman M., Woese C. "Transcriptional mapping". I. Introduction to the method and the use of actinomycin D as a transcriptional mapping agent. Proc Natl Acad Sci U S A. 1969 Jun;63(2):532–539. doi: 10.1073/pnas.63.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J., Steiner A. L., Shah V. K. Effect of molybdenum starvation and tungsten on the synthesis of nitrogenase components in Klebsiella pneumonia. J Bacteriol. 1974 Jun;118(3):986–989. doi: 10.1128/jb.118.3.986-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Postgate J. R. Genetic transfer of nitrogen fixation from Klebsiella pneumoniae to Escherichia coli. Nature. 1972 May 12;237(5350):102–103. doi: 10.1038/237102a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. Initiation, elongation and inactivation of lac messenger RNA in Escherichia coli studied studied by measurement of its beta-galactosidase synthesizing capacity in vivo. J Mol Biol. 1971 Sep 28;60(3):453–472. doi: 10.1016/0022-2836(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Mechanism of beta-galactosidase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):475–494. doi: 10.1016/0022-2836(67)90053-8. [DOI] [PubMed] [Google Scholar]
- Kepes A. Transcription and translation in the lactose operon of Escherichia coli studied by in vivo kinetics. Prog Biophys Mol Biol. 1969;19(1):199–236. doi: 10.1016/0079-6107(69)90006-6. [DOI] [PubMed] [Google Scholar]
- Khachatourians G. G., Tipper D. J. Inhibition of messenger ribonucleic acid synthesis in Escherichia coli by thiolutin. J Bacteriol. 1974 Sep;119(3):795–804. doi: 10.1128/jb.119.3.795-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. Nitrogen fixation by Klebsiella grown in the presence of oxygen. Can J Microbiol. 1972 Dec;18(12):1845–1850. doi: 10.1139/m72-288. [DOI] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parejko R. A., Wilson P. W. Regulation of nitrogenase synthesis by Klebsiella pneumoniae. Can J Microbiol. 1970 Aug;16(8):681–685. doi: 10.1139/m70-117. [DOI] [PubMed] [Google Scholar]
- Reid P., Speyer J. Rifampicin inhibition of ribonucleic acid and protein synthesis in normal and ethylenediaminetetraacetic acid-treated Escherichia coli. J Bacteriol. 1970 Oct;104(1):376–389. doi: 10.1128/jb.104.1.376-389.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Mosteller R. D., Yanofsky C. Tryptophan messenger ribonucleic acid elongation rates and steady-state levels of tryptophan operon enzymes under various growth conditions. J Mol Biol. 1970 Aug;51(3):541–550. doi: 10.1016/0022-2836(70)90007-0. [DOI] [PubMed] [Google Scholar]
- Seto B., Mortenson L. E. In vivo kinetics of nitrogenase formation in Clostridium pasteurianum. J Bacteriol. 1974 Nov;120(2):822–830. doi: 10.1128/jb.120.2.822-830.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Johnston H. M., Seidman C., Garfinkel D., Gordon J. K., Shah V. K., Brill W. J. Biochemistry and genetics of Klebsiella pneumoniae mutant strains unable to fix N2. J Bacteriol. 1975 Mar;121(3):759–765. doi: 10.1128/jb.121.3.759-765.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S. L., Gurney E. G., Valentine R. C. The nitrogen fixation genes. Nature. 1972 Oct 27;239(5374):495–499. doi: 10.1038/239495a0. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S., Gurney E., Valentine R. C. Transduction of the nitrogen-fixation genes in Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1174–1177. doi: 10.1073/pnas.68.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubb R. S. Glutamine synthetase and ammonium regulation of nitrogenase synthesis in Klebsiella. Nature. 1974 Oct 11;251(5475):481–485. doi: 10.1038/251481a0. [DOI] [PubMed] [Google Scholar]
- Tubb R. S., Postgate J. R. Control of nitrogenase synthesis in Klebsiella pneumoniae. J Gen Microbiol. 1973 Nov;79(1):103–117. doi: 10.1099/00221287-79-1-103. [DOI] [PubMed] [Google Scholar]
- Tyler B., Deleo A. B., Magasanik B. Activation of transcription of hut DNA by glutamine synthetase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):225–229. doi: 10.1073/pnas.71.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. The rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Mar 10;244(5):1128–1136. [PubMed] [Google Scholar]
- Yoch D. C., Pengra R. M. Effect of amino acids on the nitrogenase system of Klebsiella pneumoniae. J Bacteriol. 1966 Sep;92(3):618–622. doi: 10.1128/jb.92.3.618-622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


