Abstract
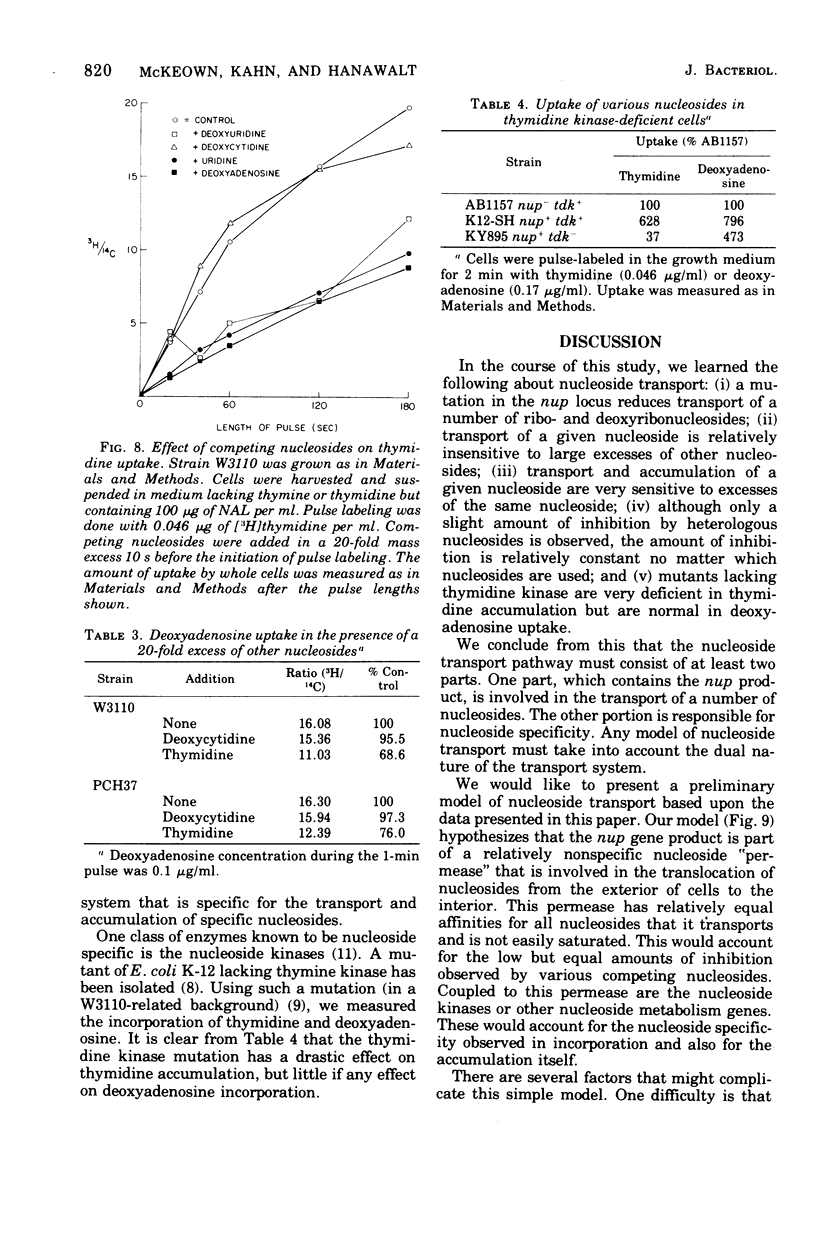
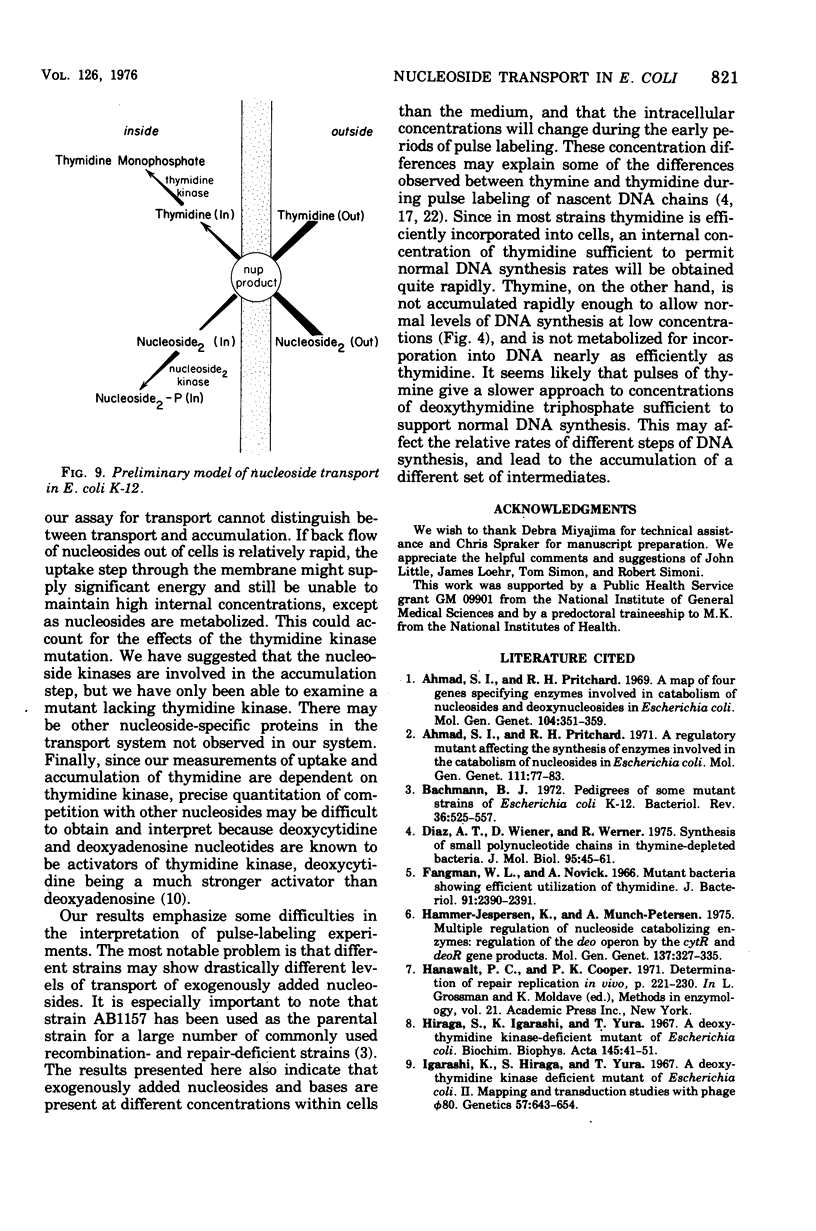
A commonly used strain of Escherichia coli K-12 was shown to be deficient in the transport of a number of nucleosides, including thymidine. Thymidine incorporation was unaffected. Strain AB2497 exhibited a strikingly lower thymidine pulse-label incorporation at low (less than 1 mug/ml) thymidine concentrations than do many other strains. The deficiency appeared to be due to mutation in a single gene. This gene, which we designated nup (for nucleoside uptake), is located at 10 to 13 min on the E. coli linkage map. In nup+ strains, the transport of a given nucleoside was relatively insensitive to large excesses of other nucleosides but was competitively inhibited by the same nucleoside. Mutants deficient inthymidine kinase are deficient in thymidine uptake but normal in deoxyadenosine uptake. A two-step model for nucleoside transport is presented in which the first step, utilizing the nup gene product, is a nonspecific translocation of nucleoside to the interior of the cell. In the second step, the individual nucleosides are modified by cellular enzymes (e.g., nucleosides kinases) facilitate accumulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S. I., Pritchard R. H. A map of four genes specifying enzymes involved in catabolism of nucleosides and deoxynucleosides in Escherichia coli. Mol Gen Genet. 1969 Aug 15;104(4):351–359. doi: 10.1007/BF00334234. [DOI] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A regulatory mutant affecting the synthesis of enzymes involved in the catabolism of nucleosides in Escherichia coli. Mol Gen Genet. 1971;111(1):77–83. doi: 10.1007/BF00286556. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A. T., Wiener D., Werner R. Synthesis of small polynucleotide chains in thymine-depleted bacteria. J Mol Biol. 1975 Jun 15;95(1):45–61. doi: 10.1016/0022-2836(75)90334-4. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Mutant bacteria showing efficient utilization of thymidine. J Bacteriol. 1966 Jun;91(6):2390–2391. doi: 10.1128/jb.91.6.2390-2391.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Ptersen A. Multiple regulation of nucleoside catabolizing enzymes: regulation of the deo operon by the cytR and deoR gene products. Mol Gen Genet. 1975;137(4):327–335. doi: 10.1007/BF00703258. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Igarashi K., Yura T. A deoxythymidine kinase-deficient mutant of Escherichia coli. I. Isolation and some properties. Biochim Biophys Acta. 1967 Aug 22;145(1):41–51. doi: 10.1016/0005-2787(67)90652-1. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hiraga S., Yura T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics. 1967 Nov;57(3):643–654. doi: 10.1093/genetics/57.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Okazaki R. Mechanism of regulation of deoxythymidine kinase of Escherichia coli. I. Effect of regulatory deoxynucleotides on the state of aggregation of the enzyme. J Mol Biol. 1967 Oct 14;29(1):139–154. doi: 10.1016/0022-2836(67)90186-6. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J., Hanawalt P. Intermediates in T4 DNA replication in a T4 ligase deficient strain. Cold Spring Harb Symp Quant Biol. 1968;33:145–150. doi: 10.1101/sqb.1968.033.01.018. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsen B. A. Regulated in vitro synthesis of the enzymes of the deo operon of Escerichia coli. properties of the DNA directed system. Mol Gen Genet. 1975;137(4):289–304. doi: 10.1007/BF00703255. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R. Mechanism of DNA replication. Nature. 1971 Apr 30;230(5296):570–572. doi: 10.1038/230570a0. [DOI] [PubMed] [Google Scholar]



