Abstract
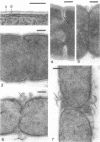
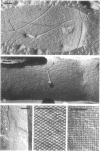
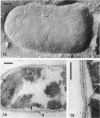
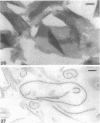
Cell walls of Clostridium thermohydrosulfuricum and C. thermosaccharolyticum have a two-layered structure, consisting of a thin, lysozyme-sensitive murein layer and a surface (S) layer composed of hexagonally or tetragonally arranged subunits. The subunits can be removed from the murein layer by treatment of cell wall preparations, are composed of a fragile, pH-sensitive monolayer of macromolecular subunits. In both organisms the first stage of the cell division process involves only the plasma membrane and the murein layer. During the subsequent cell separation, a surplus of S-layer subunits appears at the site of division, and consequently the newly formed cell poles remain completely covered by the s layer throughout the separation process. In autolyzed cells an additional layer of subunits assembles on extended areas of the inside of the mucopeptide layer. These observations indicate that the biological function of the S layer depends on its ability to maintain a complete covering of the cell surface at all stages of cell growth and division.
Full text
PDF

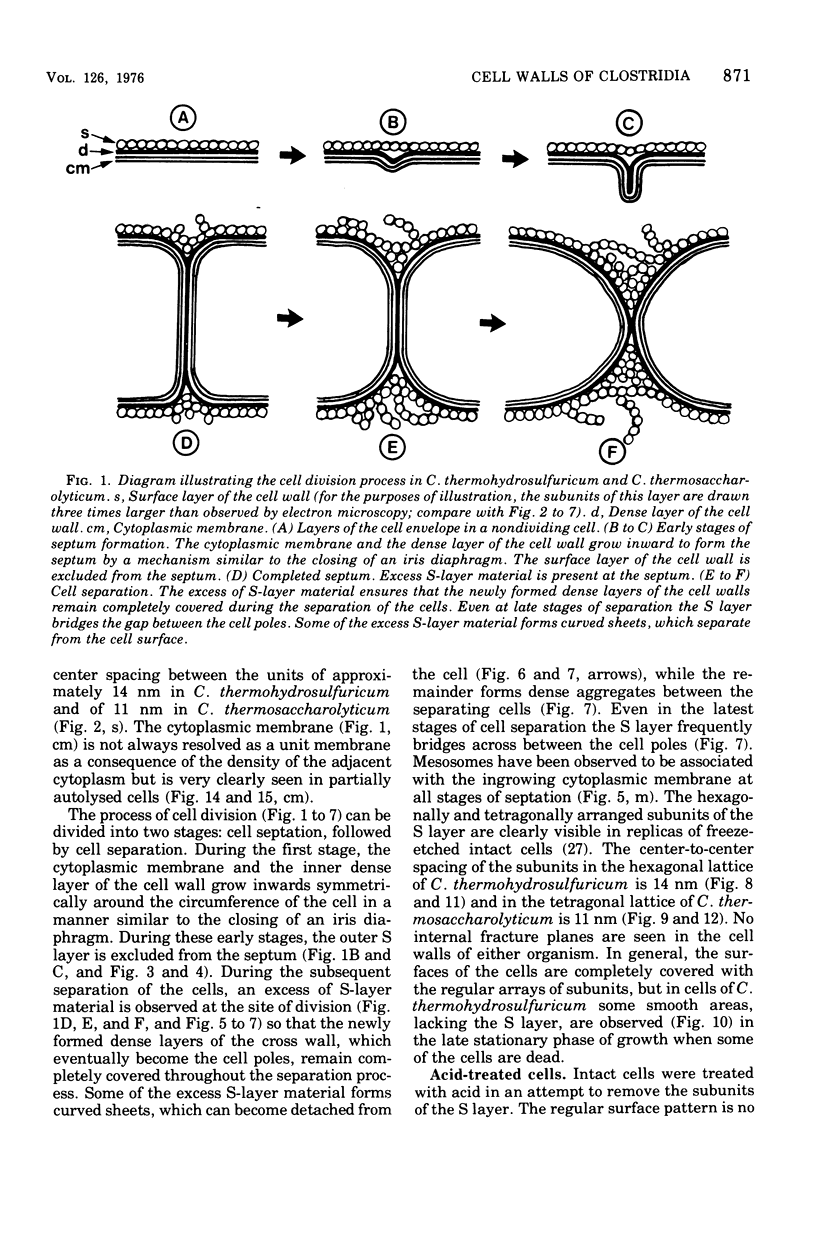
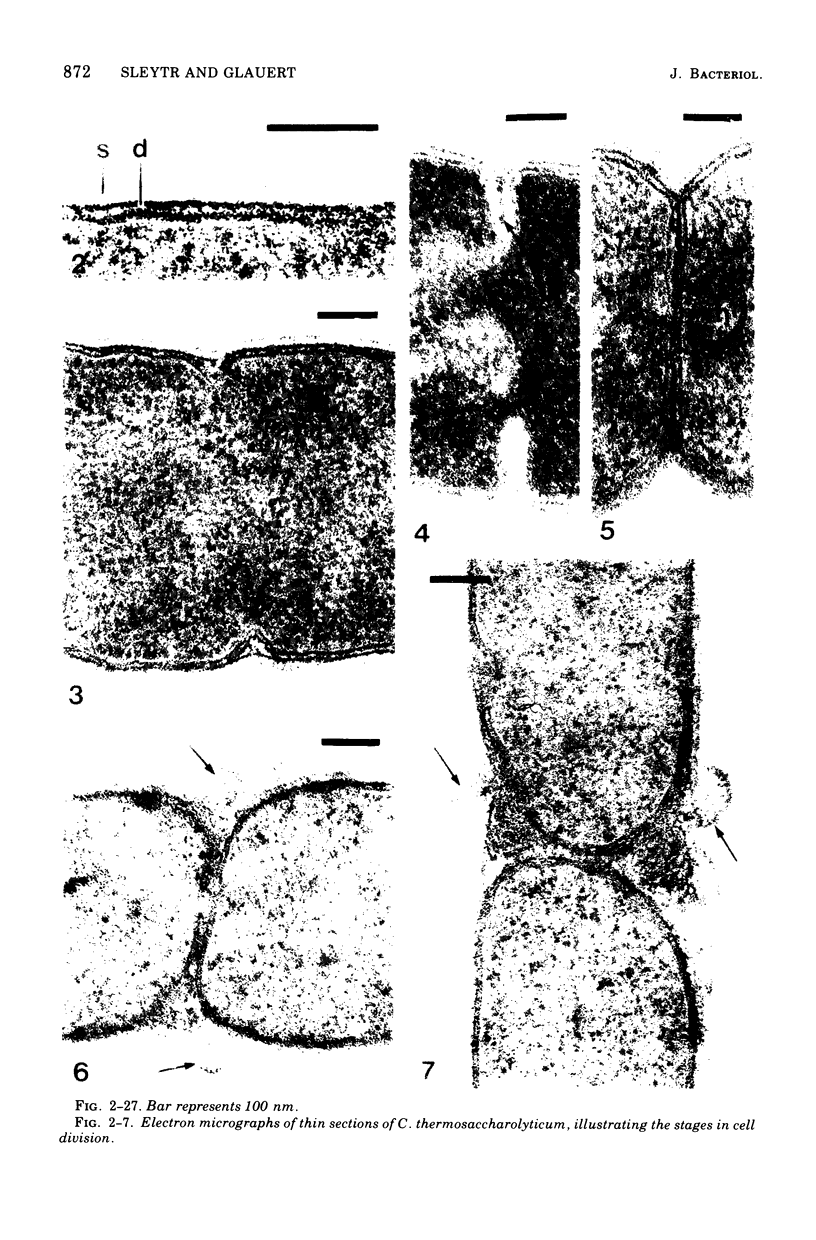





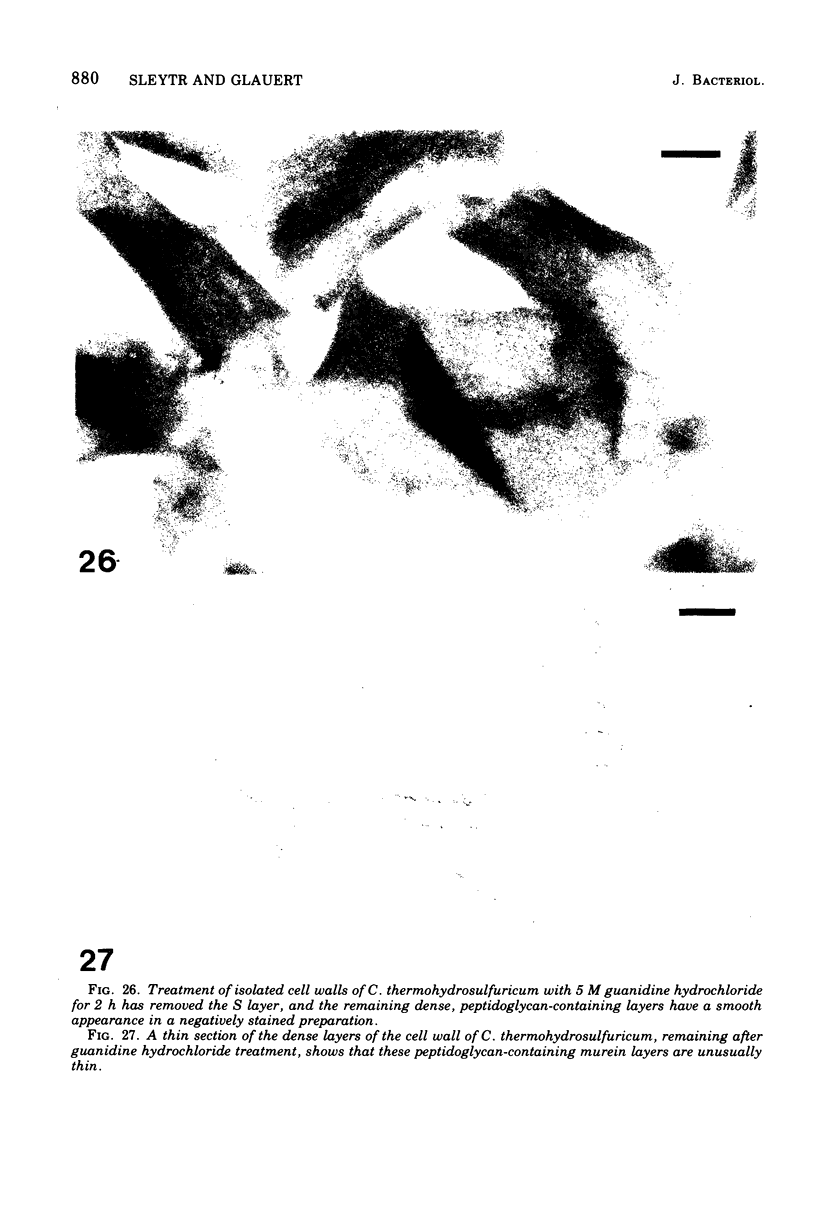


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- BADDILEY J. TEICHOIC ACIDS AND THE BACTERIAL CELL WALL. Endeavour. 1964 Jan;23:33–37. [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Orded substructure in the cell wall of Bacillus cereus. J Bacteriol. 1967 Nov;94(5):1778–1780. doi: 10.1128/jb.94.5.1778-1780.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Klug A., Nermut M. V. The structure of the macromolecular units on the cell walls of Bacillus polymyxa. J Cell Sci. 1967 Dec;2(4):587–590. doi: 10.1242/jcs.2.4.587. [DOI] [PubMed] [Google Scholar]
- Glaubert A. M., Sleytr U. B. Analysis of regular arrays of subunits on bacterial surfaces: evidence for a dynamic process of assembly. J Ultrastruct Res. 1975 Jan;50(1):103–116. doi: 10.1016/s0022-5320(75)90012-x. [DOI] [PubMed] [Google Scholar]
- Glauert A. M. Moiré patterns in electron micrographs of a bacterial membrane. J Cell Sci. 1966 Dec;1(4):425–428. doi: 10.1242/jcs.1.4.425. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
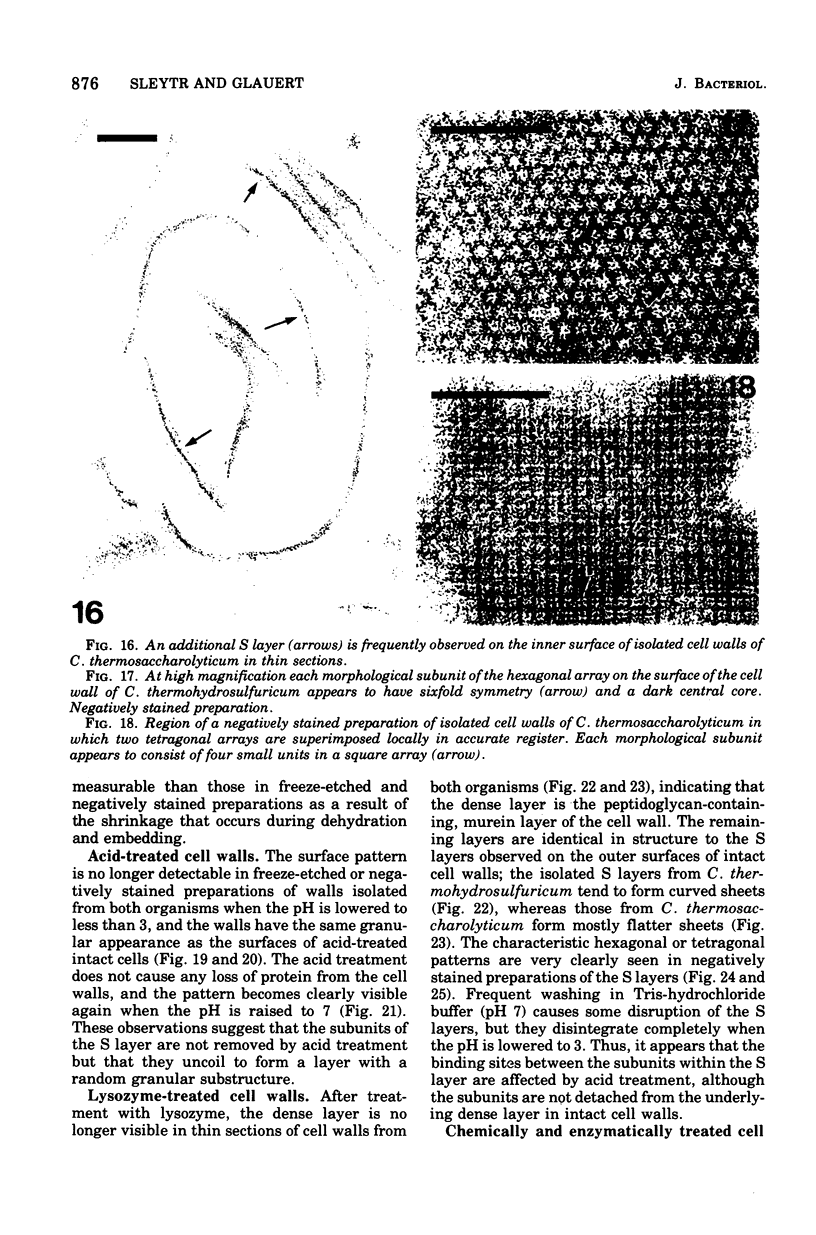
- Hollaus F., Sleytr U. On the taxonomy and fine structure of some hyperthermophilic saccharolytic Clostridia. Arch Mikrobiol. 1972;86(2):129–146. doi: 10.1007/BF00413368. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L., Tipper D. J. A polypeptide bacteriophage receptor: modified cell wall protein subunits in bacteriophage-resistant mutants of Bacillus sphaericus strain P-1. J Bacteriol. 1973 Mar;113(3):1491–1504. doi: 10.1128/jb.113.3.1491-1504.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McClung L. S. Studies on Anaerobic Bacteria: III. Historical Review and Technique of Culture of Certain Thermophilic Anaerobes. J Bacteriol. 1935 Feb;29(2):173–187. doi: 10.1128/jb.29.2.173-187.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung L. S. Studies on Anaerobic Bacteria: IV. Taxonomy of Cultures of a Thermophilic Species Causing "Swells" of Canned Foods. J Bacteriol. 1935 Feb;29(2):189–203. doi: 10.1128/jb.29.2.189-203.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B. Heterologous reattachment of regular arrays of glycoproteins on bacterial surfaces. Nature. 1975 Oct 2;257(5525):400–402. doi: 10.1038/257400a0. [DOI] [PubMed] [Google Scholar]
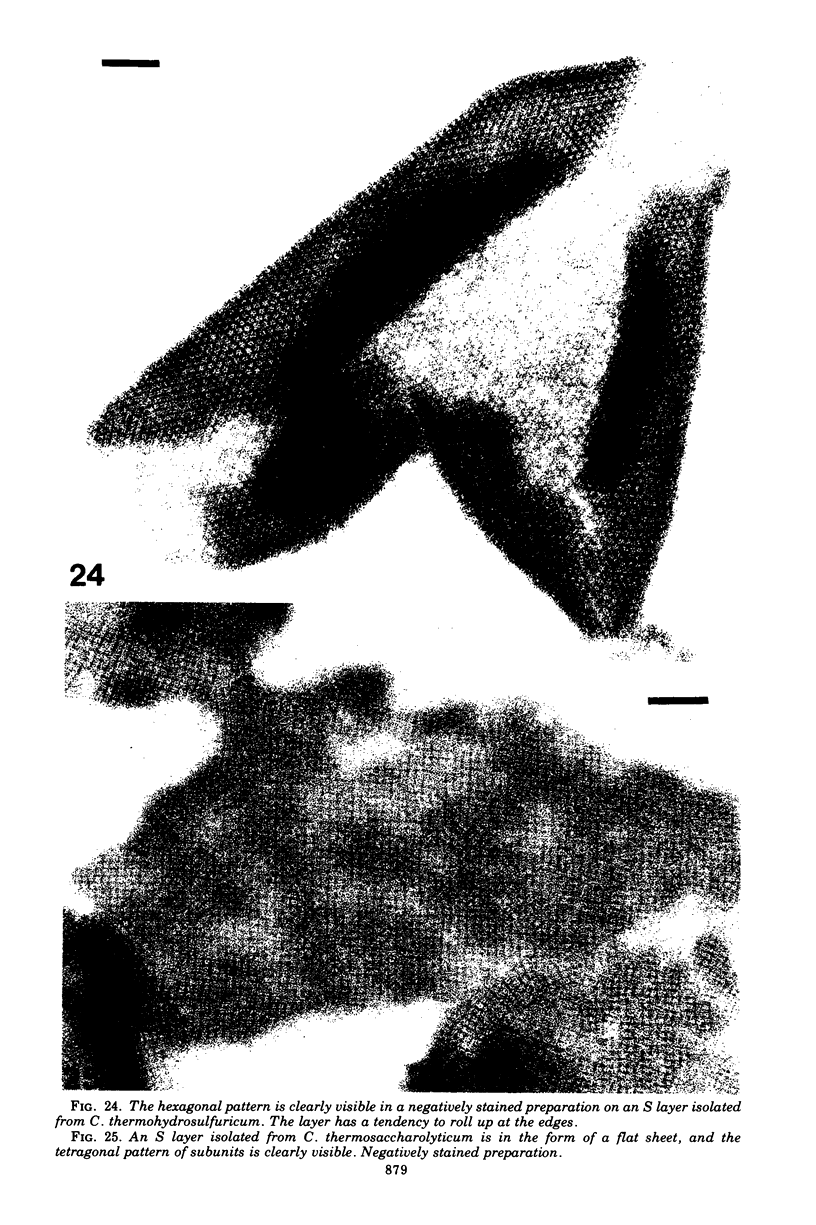
- Sleytr U. B., Kocur M., Glauert A. M., Thornley M. J. A study by freeze-etching of the fine structure of Micrococcus radiodurans. Arch Mikrobiol. 1973 Dec 4;94(1):77–87. doi: 10.1007/BF00414079. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Thornley M. J. Freeze-etching of the cell envelope of an Acinetobacter species which carries a regular array of surface subunits. J Bacteriol. 1973 Dec;116(3):1383–1397. doi: 10.1128/jb.116.3.1383-1397.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U., Adam H., Klaushofer H. Die Feinstruktur der Zellwand und Cytoplasmamembran von Clostridium nigrificans, dargestellt mit Hilfe der Gefrierätz- und Ultradünnschnittechnik. Arch Mikrobiol. 1969;66(1):40–58. [PubMed] [Google Scholar]
- Sleytr U., Adam H., Klaushofer H. Die Feinstruktur der Zellwandoberfläche von zwei thermophilen Clostridienarten, dargestellt mit Hilfe der Gefrierätztechnik. Mikroskopie. 1968 Aug;23(1):1–10. [PubMed] [Google Scholar]
- Sleytr U. Gefrierätzung verschiedener Stämme von Bacillus sphaericus. Arch Mikrobiol. 1970;72(3):238–251. [PubMed] [Google Scholar]
- Takagi A., Nakamura K., Ueda M. Electron microscope studies of the intracytoplasmic membrane system in Clostridium tetani and Clostridium botulinum. Jpn J Microbiol. 1965 Sep;9(3):131–143. doi: 10.1111/j.1348-0421.1965.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M., Sleytr U. B. Structure and assembly of bacterial surface layers composed of regular arrays of subunits. Philos Trans R Soc Lond B Biol Sci. 1974 Jul 25;268(891):147–153. doi: 10.1098/rstb.1974.0022. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Horne R. W., Glauert A. M. The fine structure of Micrococcus radiodurans. Arch Mikrobiol. 1965 Jul 20;51(3):267–289. doi: 10.1007/BF00408143. [DOI] [PubMed] [Google Scholar]
- Work E., Griffiths H. Morphology and chemistry of cell walls of Micrococcus radiodurans. J Bacteriol. 1968 Feb;95(2):641–657. doi: 10.1128/jb.95.2.641-657.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]