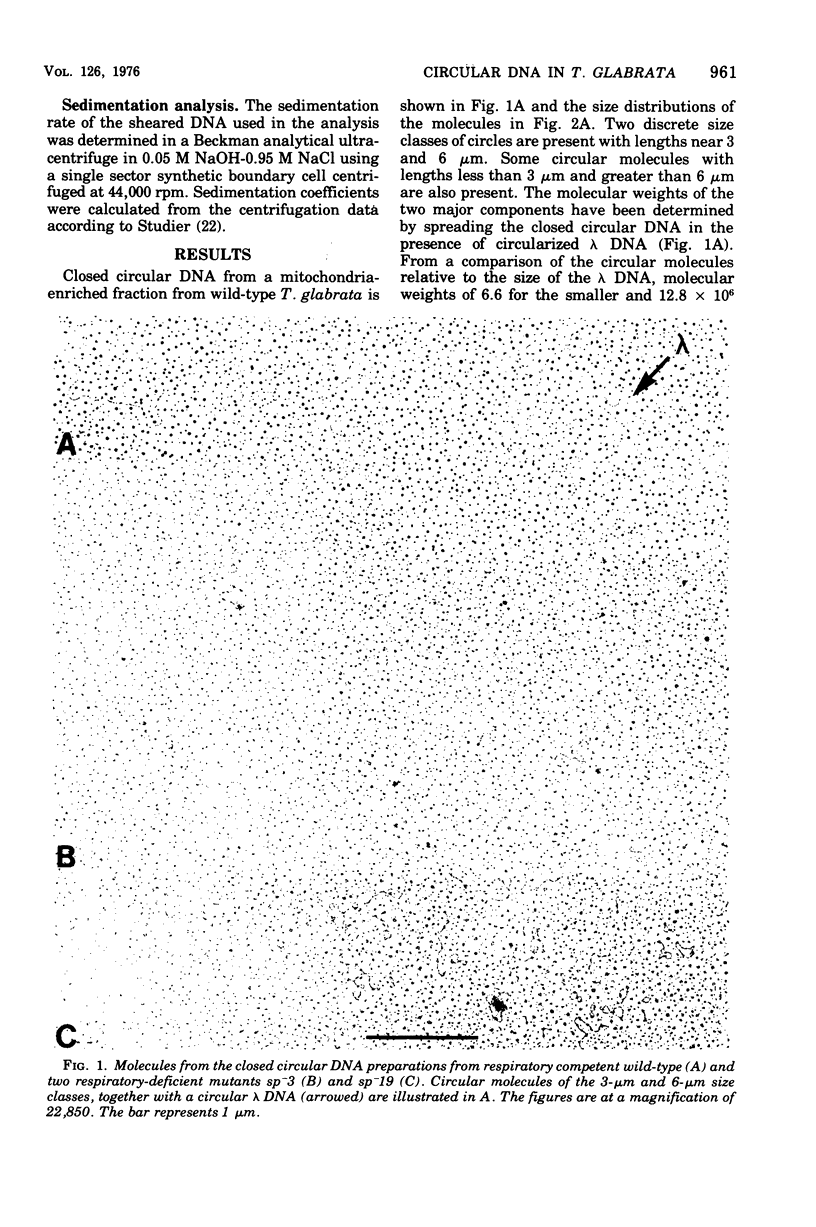
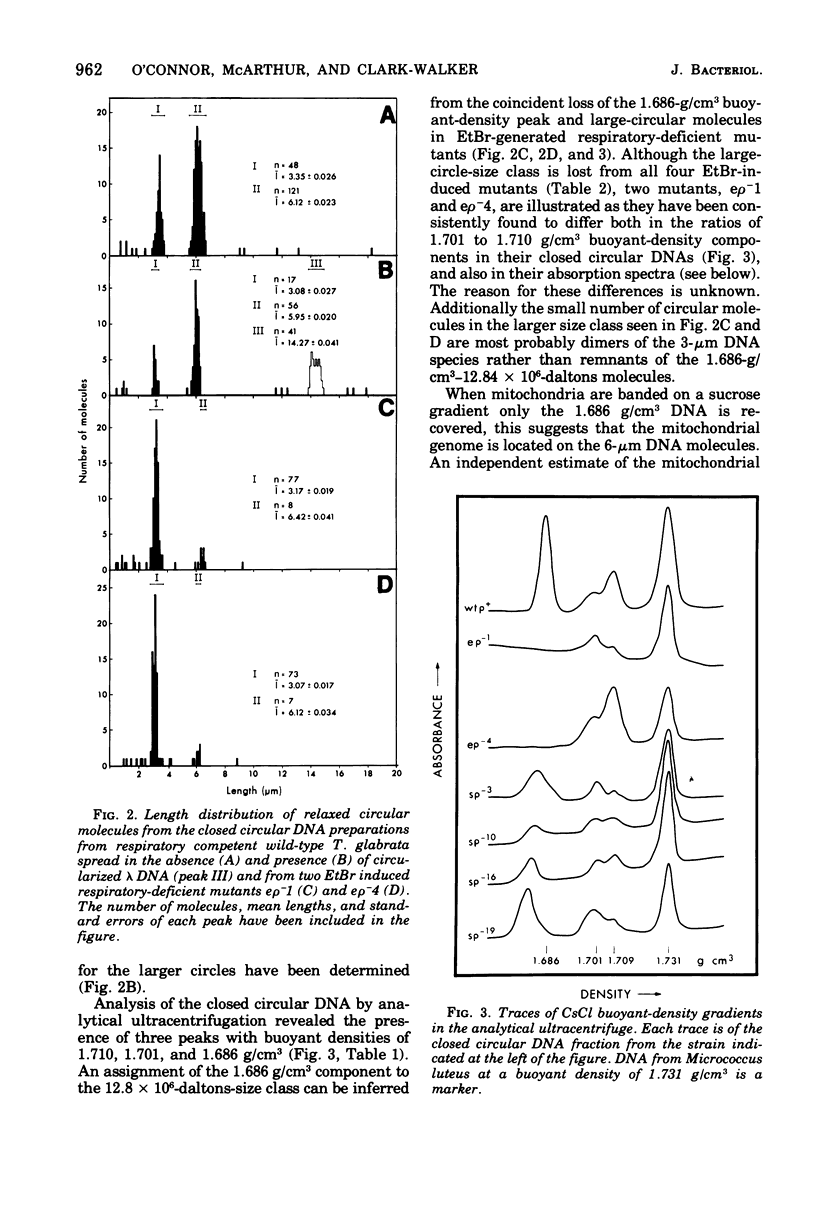
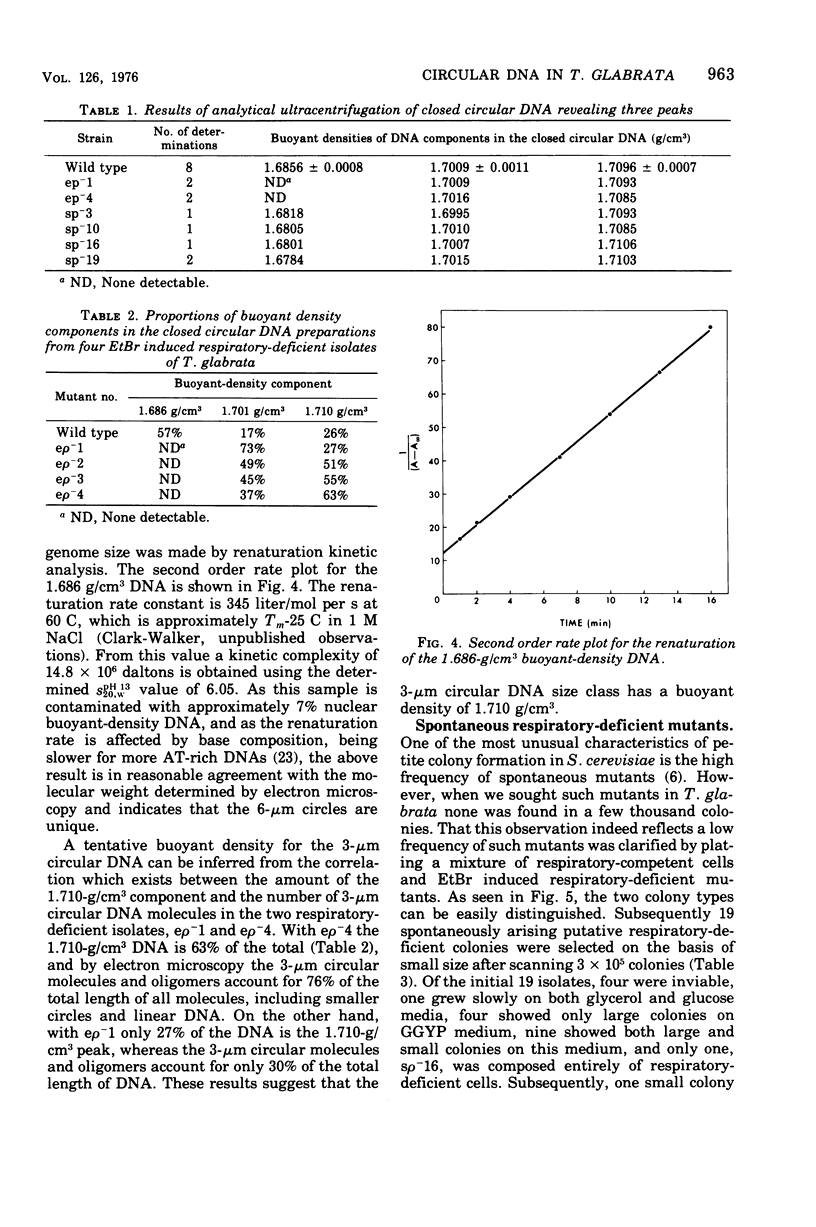
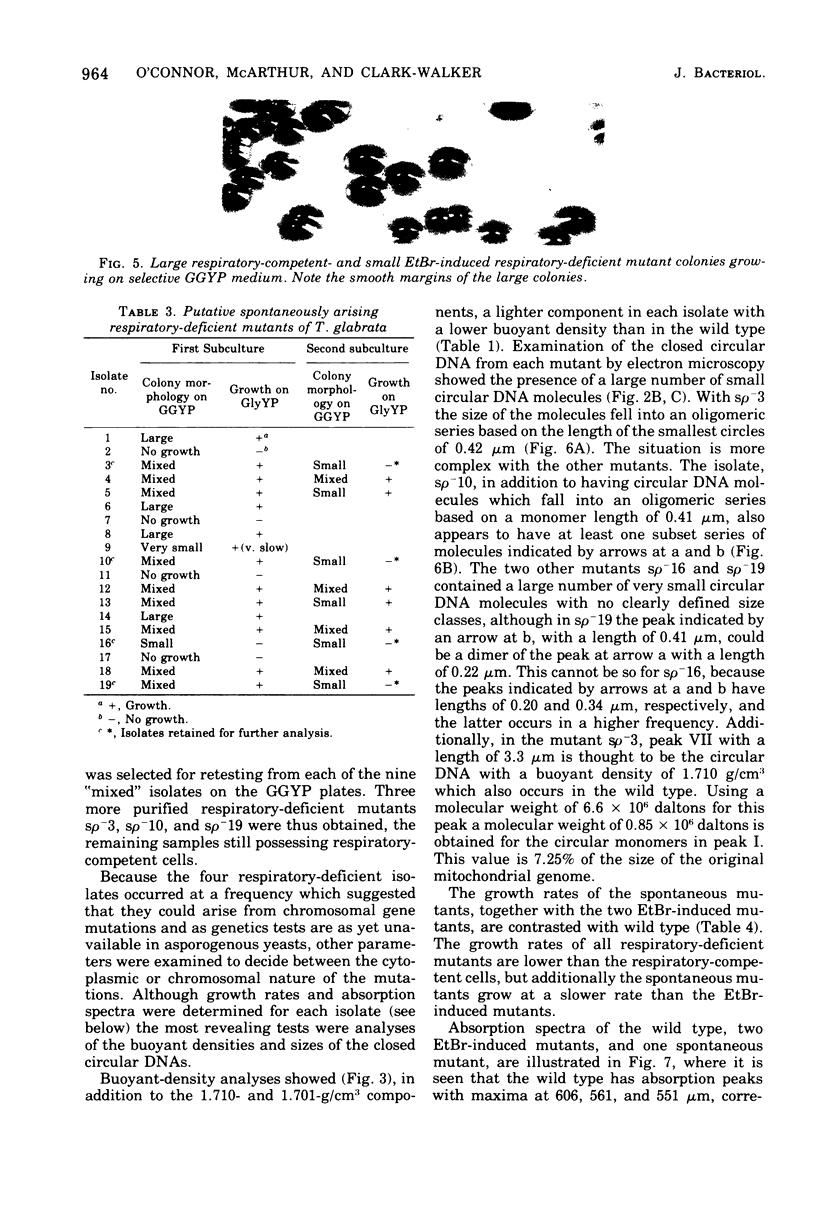
Abstract
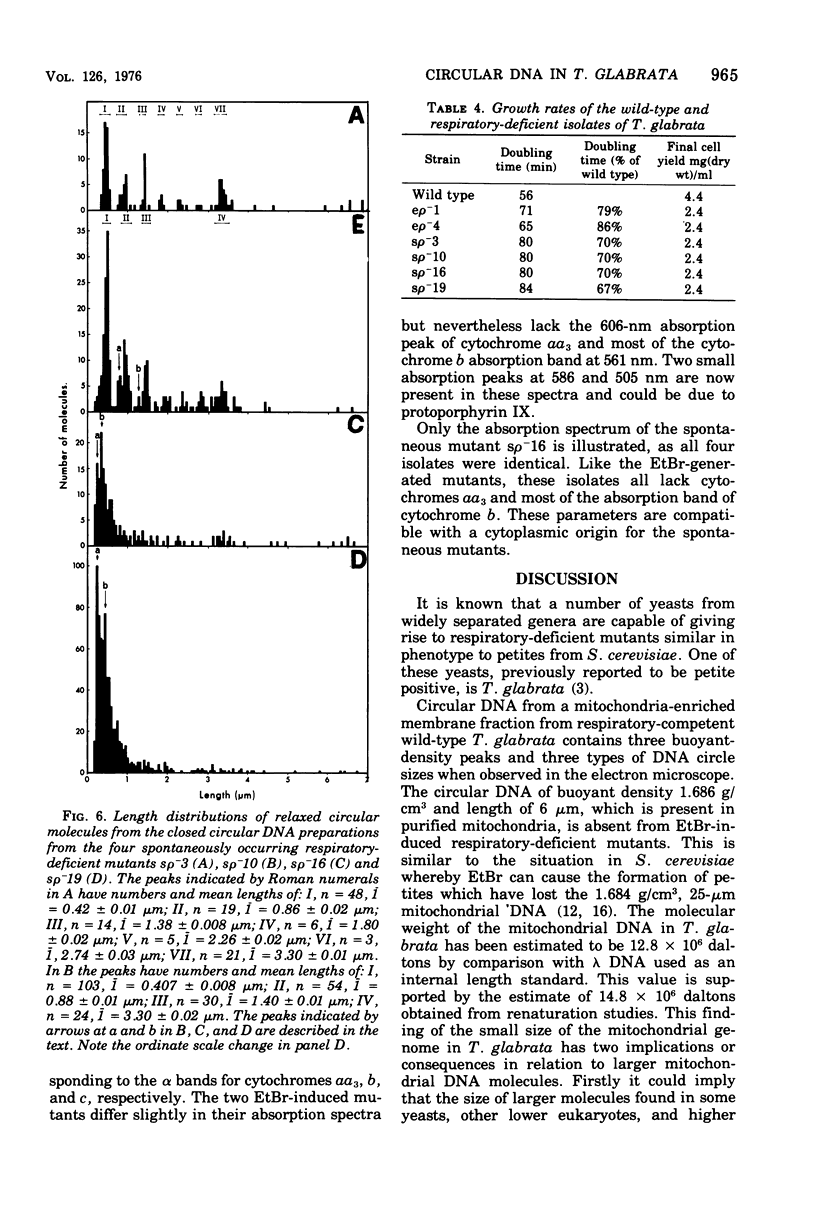
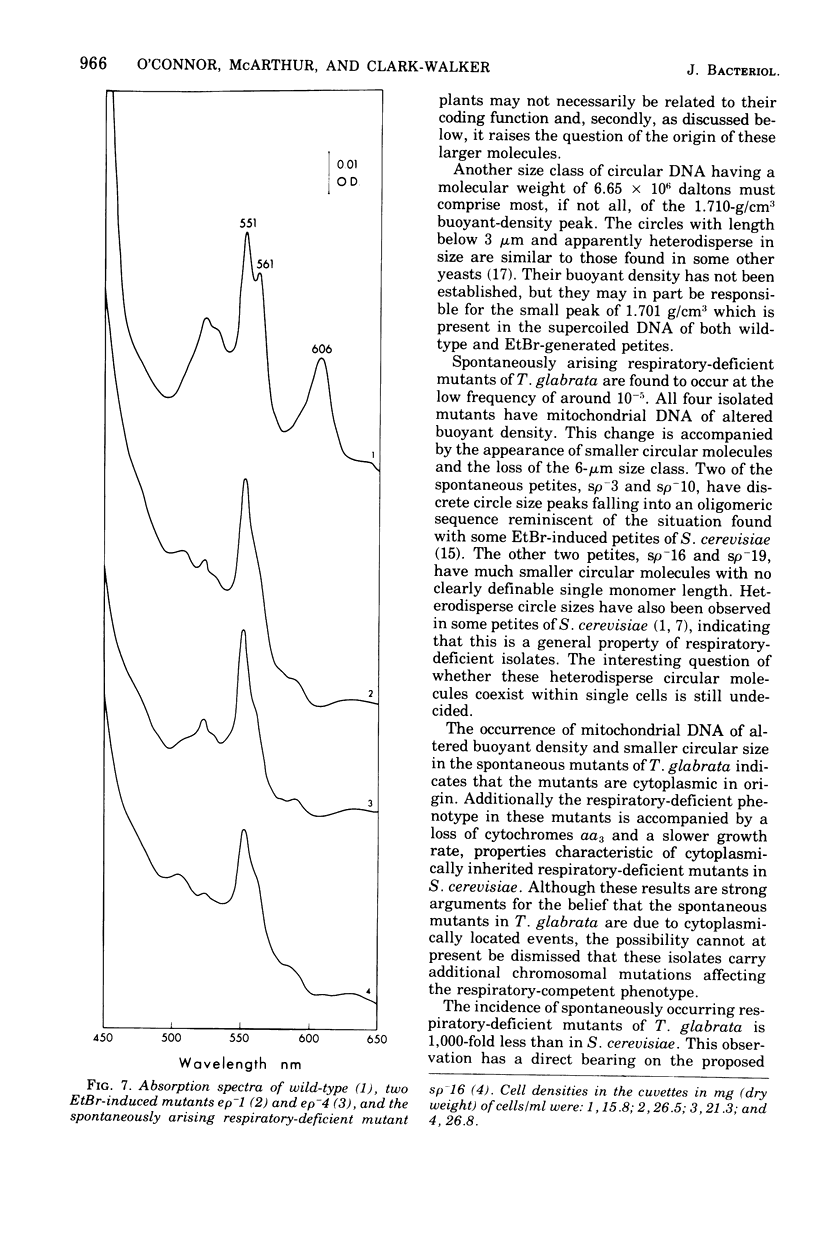
Purified mitochondria from the petite positive yeast Torulopsis glabrata contain a circular deoxyribonucleic acid (DNA) with a length of 6 mum and a buoyant density of 1.686 g/cm3. This DNA is absent from ethidium bromide induced respiratory-deficient mutants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULDER C. J. INDUCTION OF PETITE MUTATION AND INHIBITION OF SYNTHESIS OF RESPIRATORY ENZYMES IN VARIOUS YEASTS. Antonie Van Leeuwenhoek. 1964;30:1–9. doi: 10.1007/BF02046695. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Carnevali F., Nicolaieff A., Piperno G., Tecce G. Separation and characterization of a satellite DNA from a yeast cytoplasmic "petite" mutant. J Mol Biol. 1968 Nov 14;37(3):493–505. doi: 10.1016/0022-2836(68)90117-4. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Piperno G., Fonty G. The mitochondrial genome of wild-type yeast cells. I. Preparation and heterogeneity of mitochondrial DNA. J Mol Biol. 1972 Mar 28;65(2):173–189. doi: 10.1016/0022-2836(72)90275-6. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Isolation of circular DNA from a mitochondrial fraction from yeast. Proc Natl Acad Sci U S A. 1972 Feb;69(2):388–392. doi: 10.1073/pnas.69.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Complementation in cytoplasmic petite mutants of yeast to form respiratory competent cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):372–375. doi: 10.1073/pnas.72.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Localization and quantification of circular DNA in yeast. Eur J Biochem. 1974 Jan 16;41(2):359–365. doi: 10.1111/j.1432-1033.1974.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Mitochondrial genetics, circular DNA and the mechanism of the petite mutation in yeast. Genet Res. 1974 Aug;24(1):43–57. doi: 10.1017/s0016672300015068. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Size distribution of circular DNA from petite-mutant yeast lacking rho DNA. Eur J Biochem. 1973 Jan 15;32(2):263–267. doi: 10.1111/j.1432-1033.1973.tb02606.x. [DOI] [PubMed] [Google Scholar]
- DE DEKEN R. H. The dissociation of phenotypic and inheritable effects of euflavin in yeast. Exp Cell Res. 1961 Jun;24:145–148. doi: 10.1016/0014-4827(61)90256-7. [DOI] [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Huang M., Biggs D. R., Clark-Walker G. D., Linnane A. W. Chloramphenicol inhibition of the formation of particulate mitochondrial enzymes of Saccharomyces cerevisiae. Biochim Biophys Acta. 1966 Feb 21;114(2):434–436. doi: 10.1016/0005-2787(66)90330-3. [DOI] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M., Getz G. S. Electron microscopic and renaturation kinetic analysis of mitochondrial DNA of cytoplasmic petite mutants of Saccharomyces cerevisiae. J Mol Biol. 1974 Sep 15;88(2):489–507. doi: 10.1016/0022-2836(74)90497-5. [DOI] [PubMed] [Google Scholar]
- Nagley P., Linnane A. W. Mitochondrial DNA deficient petite mutants of yeast. Biochem Biophys Res Commun. 1970 Jun 5;39(5):989–996. doi: 10.1016/0006-291x(70)90422-5. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fonty G., Bernardi G. The mitochondrial genome of wild-type yeast cells. II. Investigations on the compositional heterogeneity of mitochondrial DNA. J Mol Biol. 1972 Mar 28;65(2):191–205. doi: 10.1016/0022-2836(72)90276-8. [DOI] [PubMed] [Google Scholar]
- Prunell A., Bernardi G. The mitochondrial genome of wild-type yeast cells. IV. Genes and spacers. J Mol Biol. 1974 Jul 15;86(4):825–841. doi: 10.1016/0022-2836(74)90356-8. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Heyting C., Borst P. The organization of genes in yeast mitochondrial DNA. I. The genes for large and small ribosomal RNA are far apart. Biochem Biophys Res Commun. 1975 Jul 22;65(2):699–707. doi: 10.1016/s0006-291x(75)80202-6. [DOI] [PubMed] [Google Scholar]
- Sanders J. P., Weijers P. J., Groot G. S., Borst P. Properties of mitochondrial DNA from Kluyveromyces lactis. Biochim Biophys Acta. 1974 Dec 6;374(2):136–144. doi: 10.1016/0005-2787(74)90357-8. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Zipkas D., Riley M. Proposal concerning mechanism of evolution of the genome of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1354–1358. doi: 10.1073/pnas.72.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]





