Abstract
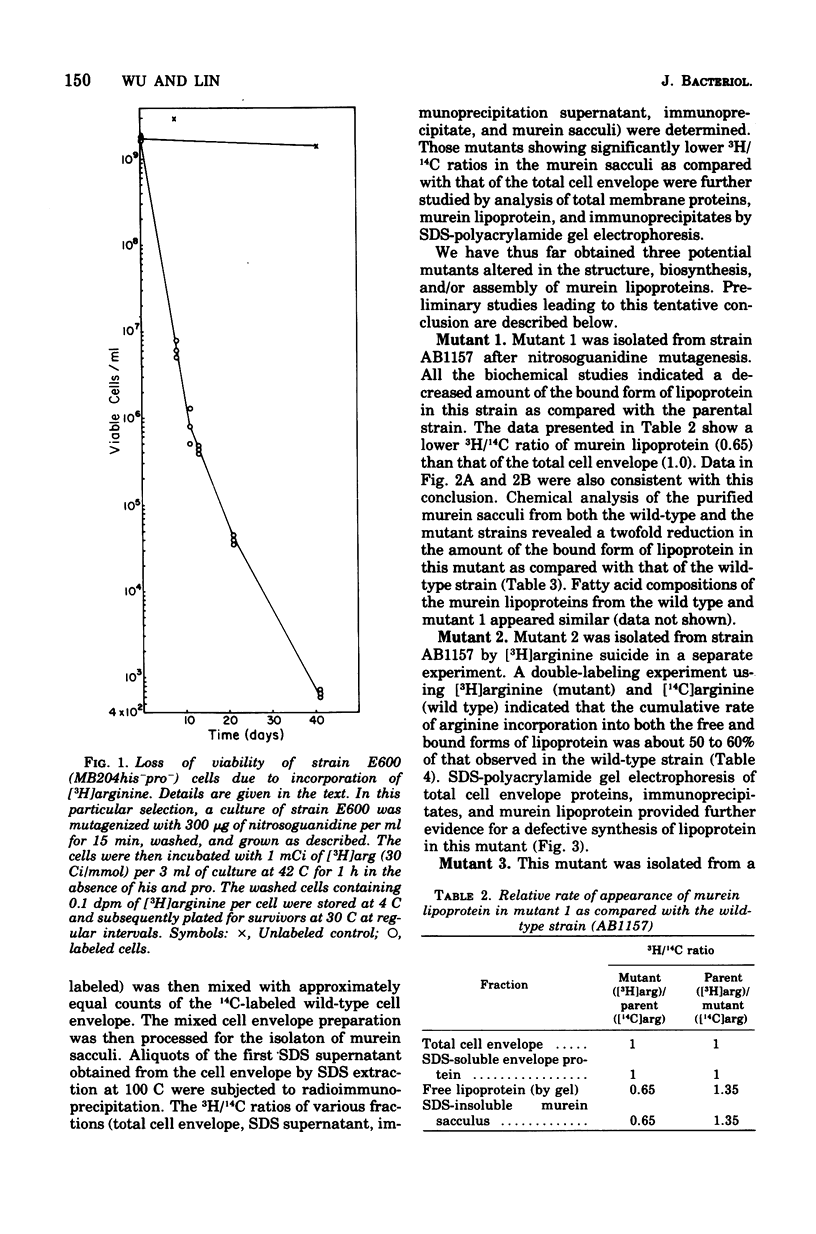
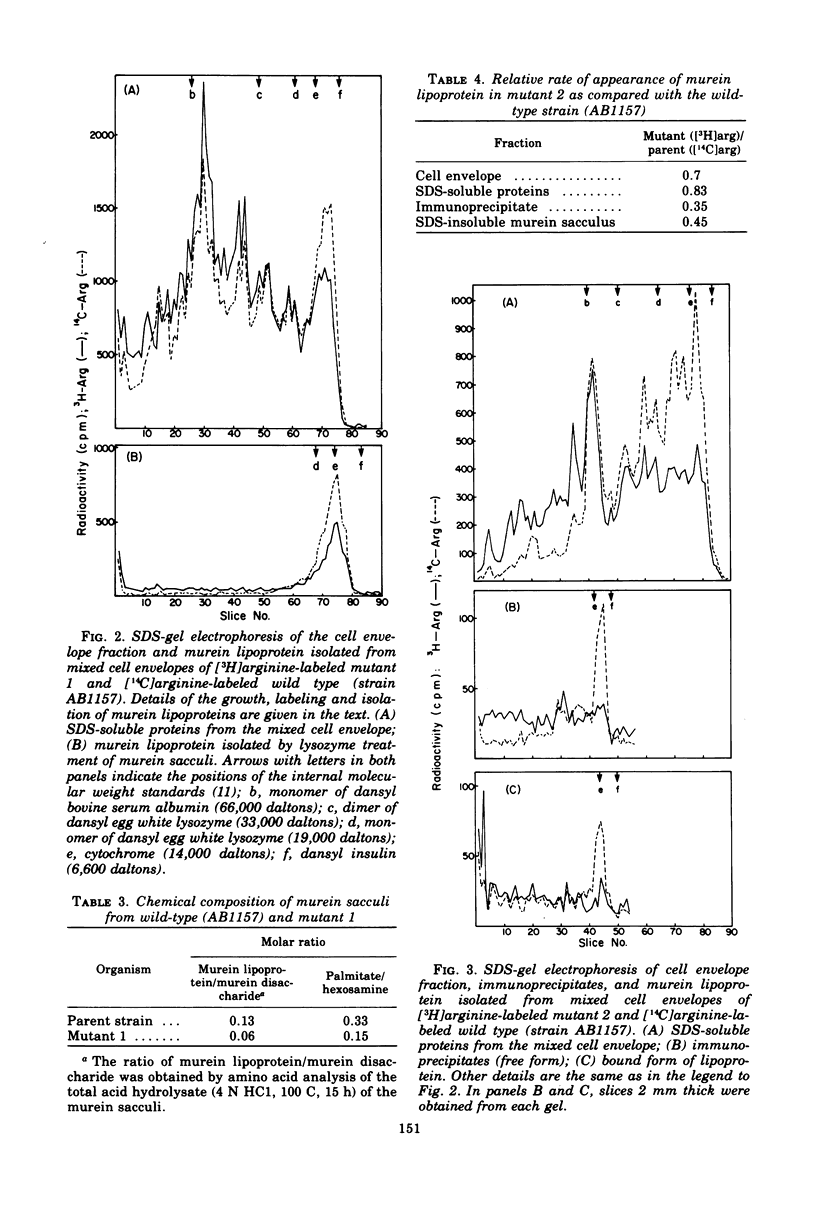
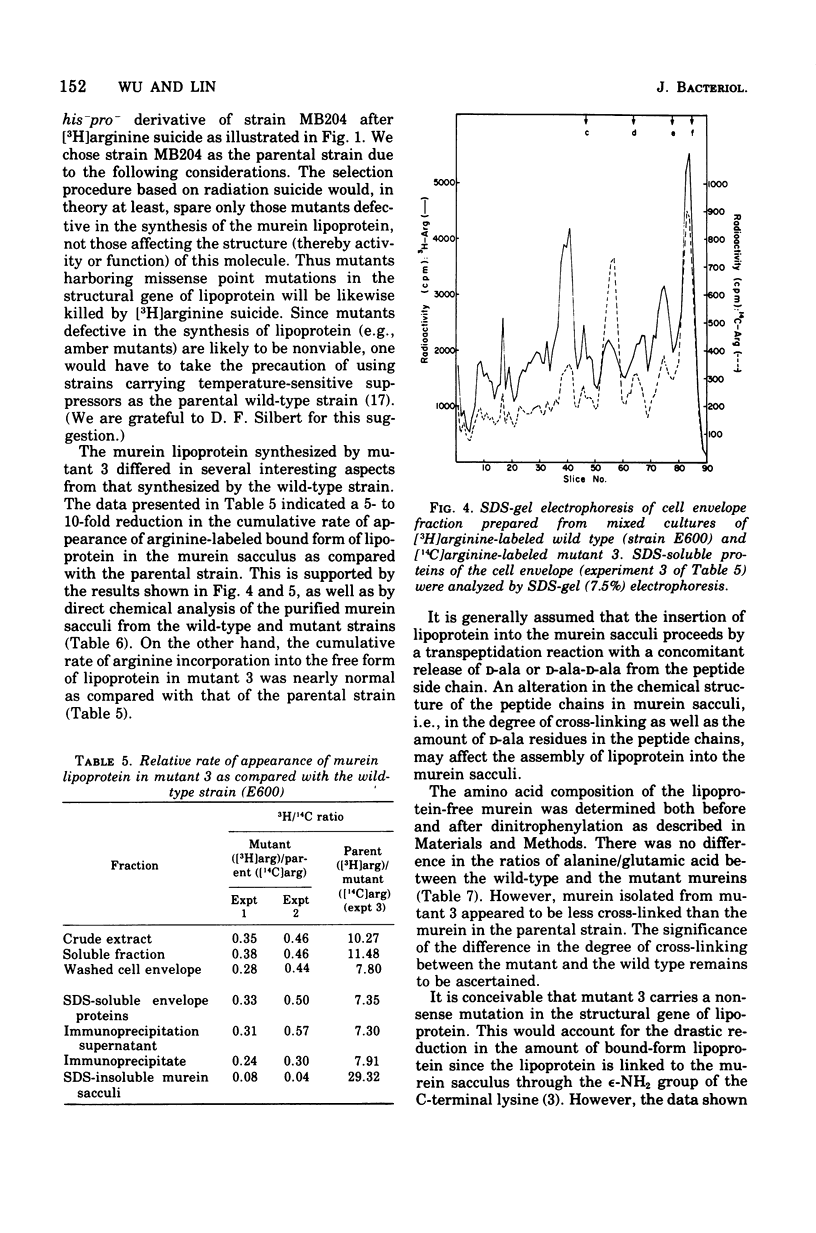
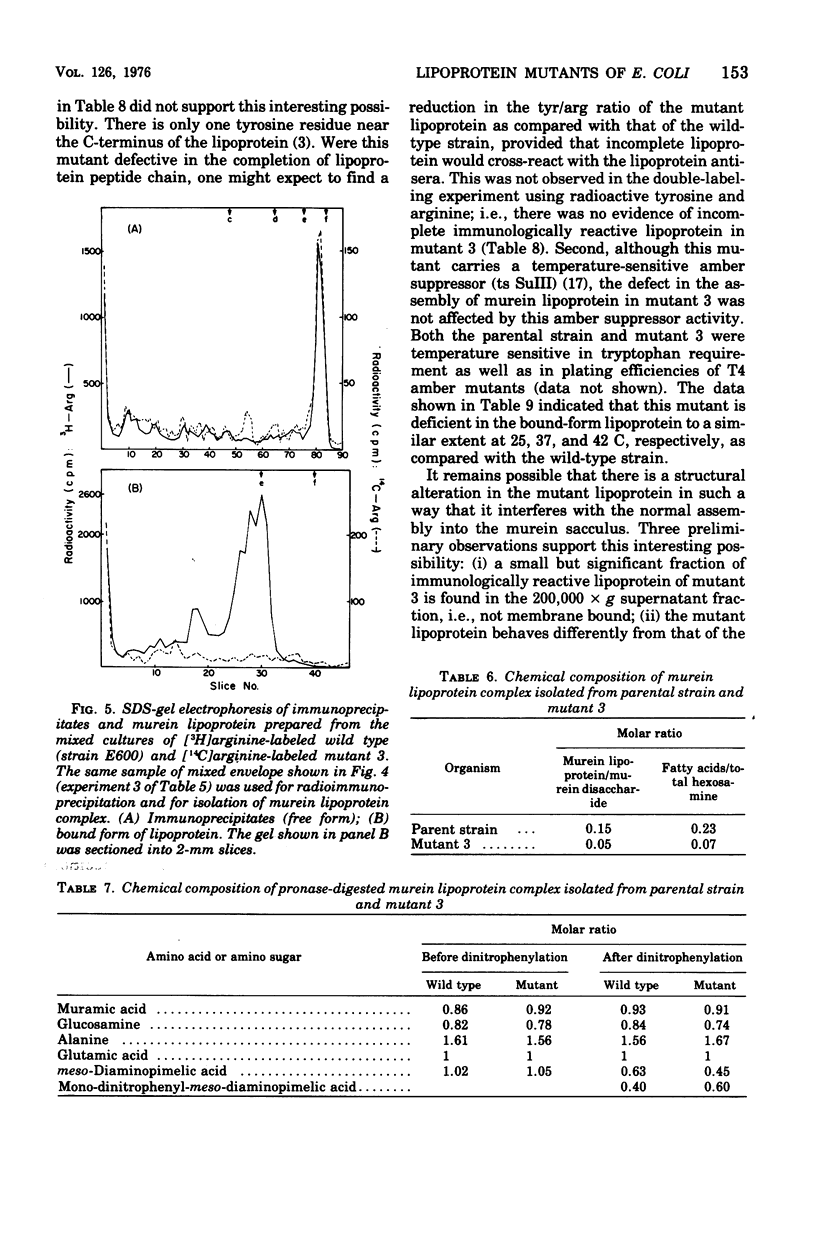
Mutants with alterations in the structure, biosynthesis, or assembly of murein lipoprotein were selected by a procedure based on radiation suicide of wild-type organisms by [3H]arginine under conditions where the radioactive arginine was preferentially incorporated into lipoprotein. Further screening for the potential mutants among the survivors of [3H]arginine suicide was carried out by using a sensitive immunodiffusion test, followed by radioactive double-labeling experiments. Three mutants were obtained and partially characterized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Bosch V. In vivo biosynthesis of murein-lipoprotein of the outer membrane of E. coli. FEBS Lett. 1973 Aug 15;34(2):302–306. doi: 10.1016/0014-5793(73)80817-8. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V. Sequence of the murein-lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972 Jun 23;28(1):51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Wang S., Inouye M. Cell-free synthesis of a specific lipoprotein of the Escherichia coli outer membrane directed by purified messenger RNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4149–4153. doi: 10.1073/pnas.71.10.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Wu H. C., Venkateswaran P. S., Inouye M. Two forms of a structural lipoprotein in the envelope of Escherichia coli. Further characterization of the free form. J Biol Chem. 1973 Aug 25;248(16):5654–5659. [PubMed] [Google Scholar]
- Inouye M. A three-dimensional molecular assembly model of a lipoprotein from the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2396–2400. doi: 10.1073/pnas.71.6.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- Lee N., Inouye M. Outer membrane proteins of Escherichia coli: biosynthesis and assembly. FEBS Lett. 1974 Feb 15;39(2):167–170. doi: 10.1016/0014-5793(74)80043-8. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Barnett L., Brenner S., Russell R. L. More mutant tyrosine transfer ribonucleic acids. J Mol Biol. 1970 Nov 28;54(1):1–14. doi: 10.1016/0022-2836(70)90442-0. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Venkateswaran P. S., Wu H. C. Isolation and characterization of a phosphonomycin-resistant mutant of Escherichia coli K-12. J Bacteriol. 1972 Jun;110(3):935–944. doi: 10.1128/jb.110.3.935-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Wu T. C. Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol. 1971 Feb;105(2):455–466. doi: 10.1128/jb.105.2.455-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



