Abstract
We have found that ectopic expression of cyclin A increases hormone-dependent and hormone-independent transcriptional activation by the estrogen receptor in vivo in a number of cell lines, including HeLa cells, U-2 OS osteosarcoma cells and Hs 578Bst breast epithelial cells. This effect can be further enhanced in HeLa cells by the concurrent expression of the cyclin-dependent kinase activator, cyclin H, and cdk7, and abolished by expression of the cdk inhibitor, p27KIP1, or by the expression of a dominant negative catalytically inactive cdk2 mutant. ER is phosphorylated between amino acids 82 and 121 in vitro by the cyclin A/cdk2 complex and incorporation of phosphate into ER is stimulated by ectopic expression of cyclin A in vivo. Together, these results strongly suggest a direct role for the cyclin A/cdk2 complex in phosphorylating ER and regulating its transcriptional activity.
The estrogen receptor (ER) is a ligand-dependent transcriptional regulatory protein that controls the genetic programs affecting many aspects of cell growth and differentiation. In addition to ligand binding, phosphorylation plays an important role in regulating ER function. The receptor contains sites for both constitutive and ligand-dependent phosphorylation. Three serine residues (amino acids 104, 106, and 118) located within the N-terminal activation domain (AF-1) and one residue in the hinge region, S294, match the consensus sequence recognized by a family of serine/threonine proline-directed kinases that includes cyclin-dependent kinases (cdk), mitogen-activated protein kinases and glycogen synthase kinase-3. Ser-104, -106, and -118 are phosphorylated upon hormone treatment; serine to alanine mutations at these positions decrease ligand-dependent transcriptional activity (1–3). Accumulating evidence suggests that mitogen-activated protein kinase can phosphorylate Ser-118 and that this may lead to estradiol-independent ER activation or, alternatively, an increase in ligand-dependent ER activation. However, whether cdks target ER as a substrate for phosphorylation and affect its transcriptional activity remains unclear. Recently, a cdk-independent effect of cyclin D1 upon ER-dependent transcriptional activity was reported in T-47D breast cancer cells (4). A link between cdk enhancement of ER function and attendant receptor phosphorylation has not yet been demonstrated.
Cdks are a family of proteins composed of a regulatory cyclin subunit associated with a catalytic kinase subunit. The cyclin subunit appears to regulate subcellular localization and timing of activation as well as substrate specificity of the kinase complex. Cdk complexes regulate the activity of target molecules, including transcriptional regulatory proteins, by phosphorylation. Regulation of cdk activity is accomplished by proteins that activate (cdk activators or CAKs), or inhibit (cdk inhibitors or CDIs), kinase function (5–8). Because cdks control cell division, the dysregulation of cyclins, their kinase partners, and/or the upstream regulatory CAKs and CDIs, have been implicated in the initiation and promotion of hyperplasia and oncogenesis. In fact, the overexpression of the regulatory cyclin subunit and the dysregulation of the catalytic cdk subunit have been identified in a number of solid tumors, leukemias, and tumor-derived cell lines (9–18).
This study examines the effects of the cyclin A/cdk2 complex on ER transcriptional activation. We chose to focus on cyclin A for several reasons: (i) Cyclin A plays a multifaceted role in cell cycle progression and is a key regulator of cdk2, a serine/threonine proline-directed kinase with the potential to phosphorylate ER. (ii) Cyclin A expression is cell adhesion-dependent, such that its overexpression can lead to adhesion-independent cell growth, a hallmark of cellular transformation (19, 20). (iii) The synthesis and degradation of cyclin A are tightly regulated, suggesting that its aberrant expression could seriously jeopardize the control of cell growth (21, 22). (iv) Cyclin A overexpression has been implicated as an important indicator of oncogenesis in several contexts including human breast tumor-derived cell lines and a mouse mammary tumor virus breast cancer model (11, 13, 23–25). (v) Cyclin A shares several features with the protooncogene cyclin D1 including the ability to bind to and phosphorylate the retinoblastoma protein, such that inappropriate cyclin A expression leads to perturbations in the regulation of the G1 to S transition (26–28). (vi) Recent reports have also linked the degradation of p27KIP1 (hereafter referred to as p27), an inhibitor of cyclin A/cdk2 activity, and aggressive breast and colorectal cancers (15, 17, 18). Together, these findings suggest that cyclin A may function as a protooncogene. To determine whether the cyclin A/cdk2 complex can affect ER function, we have examined the consequences of activation or inhibition of the cyclin A/cdk2 pathway on ER-dependent transcriptional activation.
MATERIALS AND METHODS
Plasmids.
A FLAG epitope was added to the N terminus of the full-length wild-type ER cDNA. This construct was inserted into the pCMV-Neor (Invitrogen) expression vector; 0.5 μg DNA per 60-mm dish was used in the transfections. The vector pCDLSRα296 was used to express cyclin A, cyclin H, cdk7, cdk2, or the dominant negative mutant, cdk2TS. The pCMV5 plasmid expressed p27. Two micrograms of cyclin or cdk DNA was used for each 60-mm transfection plate, except in Fig. 2C where the amount of cyclin A-encoding plasmid was varied from 0.5 to 10.0 μg per dish. The ΔETCO reporter plasmid contained one estrogen response element upstream of the thymidine kinase promoter (−109) driving the expression of the chloramphenicol acetyltransferase (CAT) gene. This reporter lacks a nearby activator protein-1 binding site to ensure that the results obtained are not influenced by other regulatory elements in the plasmid. The vector pCMV-lacZ was used as an internal control to measure the efficiency of each transfection. The reporter- and β-galactosidase (β-gal)-encoding vectors were used at 2.0 and 0.5 μg DNA per 60-mm dish, respectively.
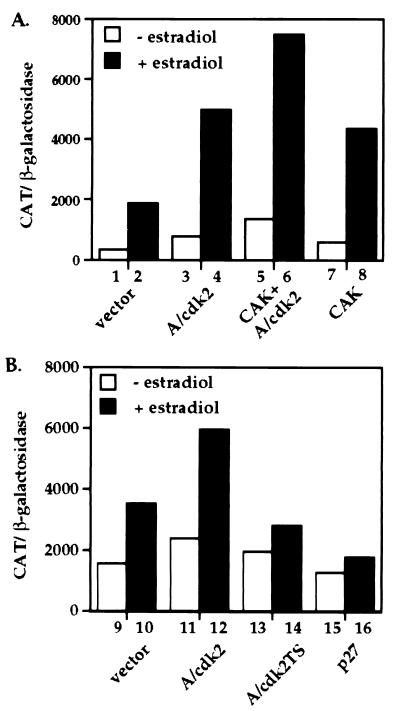
Figure 2.
Effects of cdk activators and inhibitors on ER transcriptional enhancement. (A) Effects of CAK (cyclin H/cdk7) on ER transcriptional activation. HeLa cells were transiently transfected using the calcium phosphate procedure with paired ER expression and reporter plasmids as described in Fig. 1, along with a control empty expression vector, (lanes 1 and 2); or expression vectors for cyclin A/cdk2 (lanes 3 and 4); cyclin A/cdk2 + CAK (lanes 5 and 6) and CAK alone (lanes 7 and 8). (B) Effects of cdk inhibitors on ER transcriptional activation. HeLa cells were transiently transfected with ER expression and reporter constructs along with an empty expression vector (lanes 9 and 10); or expression vectors for cyclin A/cdk2 (lanes 11 and 12); cyclin A/cdk2TS (dominant negative) (lanes 13 and 14) and p27 (lanes 15 and 16). Hormone treatment and activity assays were performed as described in Fig. 1. Data represent the mean of two experiments done in duplicate with <10% error.
Mammalian Cell Culture and Treatments.
The cell lines used in these studies were obtained from the American Type Culture Collection and maintained in DMEM (GIBCO/BRL) supplemented with 10% fetal bovine serum (HyClone), 50 units/ml each of penicillin and streptomycin (GIBCO/BRL), and 2 mM l-glutamine (GIBCO/BRL). Transfections were performed in phenol red-free DMEM supplemented with 10% charcoal-stripped fetal bovine serum. For transfections, cells were seeded into 60-mm dishes at 2 × 105 cells per dish and transfected the following day by either the calcium phosphate precipitation or the liposome-mediated (Lipofectamine, GIBCO/BRL) methods (29). At 12–16 h posttransfection, cells were rinsed twice with PBS and refed with phenol red-free DMEM supplemented with 10% charcoal-stripped fetal bovine serum containing either 100 nM 17β-estradiol or the ethanol vehicle. CAT and β-gal assays were performed 24 h later as described (30). Protein expression was monitored by preparing whole cell extracts. Cells were lysed for 30 min on ice in 200 μl of high salt lysis buffer [400 mM NaCl/50 mM Tris⋅HCl, pH 8.0/0.5% Nonidet P-40/1 mM EDTA/1 mM DTT with protease inhibitors (1 μg/ml aprotinin/1 μg/ml leupeptin/1 μg/ml pepstatin A/1 mM phenylmethylsulfonyl fluoride)] and phosphatase inhibitors (1.0 mM NaF/10 mM β-glycerophosphate/1.0 mM sodium orthovanadate). Whole cell extract (100 μg) was separated by SDS/10% polyacrylamide gel and transferred to Immobilon paper (Millipore).
Glutathione S-Transferase (GST)-Protein Expression and Purification.
Truncated versions of the human ER cDNA coding for amino acids 1–82, 1–115, and amino acids 1–121 were cloned into pGEX-5T-1 (Pharmacia). GST fusion proteins were expressed and purified as described (31).
Insect Cell Culture and Baculovirus Methods.
High Five insect cells were maintained in Ex-Cell 405 Insect Culture Media (JRH Biosciences, Lenexa, KS) at 27°C. Baculovirus vectors (10−7 plaque-forming units) engineered to express human cyclin A or an hemagglutinin-tagged human cdk2 were used separately or in combination to infect cells. Cells (1 × 107cells per 100-mm dish) were infected with 0.5 ml of virus in a final volume of 3.0 ml for 2 h at 27°C and refed with 10 ml of Ex-Cell medium. Two days postinfection, cells were lysed on ice for 30 min in 0.5 ml of 120 mM NaCl, 50 mM Tris⋅HCl (pH 8.0), 0.5% Nonidet P-40, 1 mM EDTA, 1 mM DTT with protease and phosphatase inhibitors as described above.
Immunoprecipitations.
Insect cell immunoprecipitations were performed using ≈100 μg of extract and 5 μg of the mAb 12CA5 (Boehringer Mannheim) directed against the cdk hemagglutinin-epitope, or 5 μg of a human cyclin A-specific polyclonal antibody (#06–138, Upstate Biotechnology, Lake Placid, NY). Immune complexes were immobilized on protein A/G agarose beads (Santa Cruz Biotechnology), washed four times in 0.5 ml of lysis buffer and used in the in vitro kinase assay.
In Vitro Kinase Assays.
The GST-ER substrate (10 μg), ER 1–82, ER 1–115, or ER 1–121, was absorbed to 100 μl of a 50% slurry of glutathione-Sepharose 4B beads (Pharmacia) for 20 min at room temperature and washed twice with kinase buffer (50 mM potassium phosphate, pH 7.15/10 mM MgCl2/5 mM NaF/4.5 mM DTT/1 mM phenylmethylsulfonyl fluoride). The immobilized substrate was added to the immunopurified kinase subunit(s) and incubated on ice for 5 min prior to the initiation of the kinase reaction in a final volume of 150 μl as described (31). The reaction products were separated by 12.5% SDS/PAGE, stained with Coomassie blue to visualize the receptor band, and autoradiography was performed from 5 to 30 min at room temperature. Aliquots of the reaction mixtures were also separated by SDS/PAGE and subjected to Western blot analysis to determine the levels of ER, cyclin A, and cdk2.
In Vivo Metabolic Labeling.
HeLa cells (1 × 106 cells per 100-mm dish) were transiently transfected with FLAG-ER and/or cyclin A and metabolically labeled with 1 mCi/ml (1 Ci = 37 GBq) of [32P]orthophosphate in 2 ml of phosphate-free DMEM for 2 h at 37°C in the absence or presence of 100 nM 17β-estradiol. Cells were washed twice with PBS, placed on ice, and lysed directly on the plate by adding 200 μl of high salt lysis buffer. The in vivo labeled FLAG-tagged ER was immunopurified using 5 μg of the monoclonal anti-FLAG antibody (M2, Eastman Kodak). The ER protein recovered by immunoprecipitation was resolved on SDS/10% polyacrylamide electrophoresis gel, silver-stained, and dried. Autoradiography was performed for 12 h at room temperature to visualize the radiolabeled ER. The incorporated radioactivity was quantified using the National Institutes of Health image program to analyze the scanned autoradiogram and a digitized version of the silver stained gel.
RESULTS
Increased ER Transcriptional Enhancement by Ectopic Cyclin A Expression.
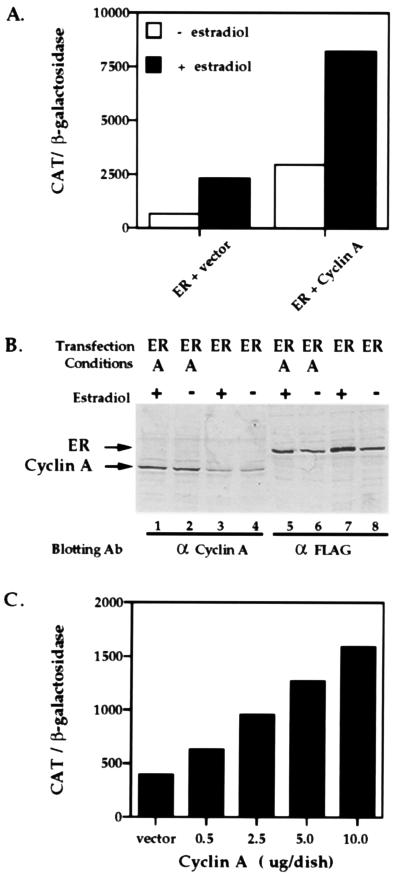
To establish whether ectopic expression of cyclin A affects ER-dependent activation, we examined the ability of cyclin A to increase ER-mediated transcriptional enhancement. ER-deficient HeLa cells were transfected with an expression vector for the full-length human ER containing a FLAG epitope at its N terminus, the reporter plasmid estrogen response element-thymidine kinase-CAT, plasmids encoding human cyclin A and a constitutive β-gal expression vector as an internal transfection standard. Transfected cells were treated with 17β-estradiol or the ethanol vehicle for 24 h. Transcriptional activity was measured by CAT assay and normalized to β-gal activity. As shown in Fig. 1A, both hormone-dependent and hormone-independent ER transcriptional activity were increased roughly 3-fold when cyclin A is overexpressed. No effect of cyclin A on reporter gene activity was observed in the absence of ER (not shown). To ensure that this increased transcriptional activity was not a result of additional ER protein production, we monitored protein expression in whole cell extracts using Western blot analysis. As Fig. 1B illustrates, ER levels are not increased by cyclin A coexpression (compare lanes 5 and 6 to lanes 7 and 8). In addition, cyclin A is expressed above endogenous levels as a result of our transient transfection scheme and estradiol treatment does not alter cyclin A expression (Fig. 1B, compare lanes 1 and 2 to lanes 3 and 4). By increasing the amount of cyclin A used in these transfections, we were able to observe a concomitant increase in ER transcriptional activation (Fig. 1C). Coexpression of cyclin A and cdk2 also results in an increased ER-dependent transcriptional activity slightly above that of cyclin A alone. Expression of cdk2 alone, on the other hand, did not significantly alter the ER-dependent transcriptional activity (not shown). These findings suggest that cyclin A is a limiting factor for full hormone-dependent ER-mediated transcriptional enhancement, presumably by favoring the formation of active cyclin/cdk complexes from endogenous cdk2 subunits. Thus, cyclin A expression greatly magnifies the characteristic hormone-dependent ER transcriptional response, which suggests that this cyclin/cdk complex can act as an effector of the ER signaling pathway.
Figure 1.
Activation of ER transcriptional enhancement by ectopic cyclin A expression. (A) ER-deficient HeLa cells (2 × 105 cells per 60-mm dish) were transiently transfected using Lipofectamine with 2 μg of the estrogen response element containing reporter plasmid, possessing a single estrogen response element upstream of the thymidine kinase promoter fused to the CAT gene (ΔETCO), and 0.5 μg of the ER expression vector and 4 μg of the expression vector SRα296 (ER + vector) or 0.5 μg of the ER expression vector and 4 μg of SRα296-cyclin A (ER + cyclin A), along with 0.5 μg of pCMV-lacZ as an internal standard for transfection efficiency. Cells were incubated with 100 nM 17β-estradiol or the ethanol vehicle for 24 h as indicated, harvested and assayed for CAT and β-gal activity. (B) ER and cyclin A expression in transfected HeLa cells. Whole cell extracts were prepared from a parallel set of transfected cells. Equal amounts of protein (100 μg per lane) were separated by SDS/10% polyacrylamide gel, transferred to Immobilon paper, probed with the M2 monoclonal antibody directed against the FLAG-epitope on ER or a polyclonal antibody against human cyclin A, and visualized with an alkaline phosphatase-conjugated goat secondary antibody. (C) Increasing amounts of cyclin A lead to increased ER transcriptional activity. Using the calcium phosphate procedure, HeLa cells were transiently transfected with increasing amounts of cyclin A (0.5 μg to 10.0 μg) with a constant amount of ER expression and reporter plasmids, and CAT activity was measured in the presence of 17β-estradiol. For transfection experiments, data represent the mean of at least two experiments done in duplicate with <10% variation.
Reciprocal Effects of cdk Activators and Inhibitors on ER Transcriptional Enhancement.
To further demonstrate that alterations in cyclin A/cdk2 activity can modify ER transcriptional enhancement, we used two classes of cdk regulatory proteins, CAK, which is composed of cyclin H and cdk7, and the CDI, p27. p27 inhibits many cyclin/cdk complexes, including cyclin A/cdk2, cyclin E/cdk2, and cyclin D/cdk4 (32, 33). We were particularly interested in studying p27 in light of recent reports (15, 17, 18) linking its premature or excessive degradation to aggressive breast and colorectal cancers.
Fig. 2A illustrates that cdk activation by expression of cyclin A/cdk2 or CAK (cyclin H and cdk7) leads to a greater than 2-fold increase in both hormone-dependent and hormone-independent ER transcriptional activity. The coexpression of all four proteins, the cyclin/cdk complex as well as the CAK complex, further augments (4-fold) this response and lends further support for cyclin/cdk involvement in the regulation of ER-dependent transcriptional activity.
We next asked if a decrease in cdk activity would reduce ER-dependent transcriptional activation. We chose two means of inhibiting cdk2 activity. Initially, the CDI, p27, was ectopically expressed in HeLa cells and ER-dependent transcriptional enhancement was measured. Ligand-dependent and, to a lesser degree, ligand-independent transcriptional activation by ER was reduced by p27 expression (Fig. 2B). This effect is noted in either the presence or absence of ectopically expressed cyclin A. Therefore, reducing cdk activity leads to impaired ER transcriptional activity.
At this point, we could not discriminate between an effect of p27 upon cdc2, cdk2, or cdk4, since p27 can inhibit all of these kinases. Therefore, we sought another means of reducing cdk2 activity by using a catalytically inactive cdk2 mutant to specifically block endogenous cdk2 activity. This cdk derivative, designated cdk2TS, is competent for cyclin A binding, but it cannot bind to ATP due to two consecutive amino acid changes in the ATP-binding site (Lys-33 and -34 are replaced by threonine and serine, respectively). This mutant acts as a dominant negative by sequestering cyclin A, thereby preventing it from binding and activating endogenous wild-type cdk2.
By expressing the dominant negative cdk2 mutant, we were able to reduce significantly the ER response to ligand treatment (Fig. 2B). Ectopic expression of a dominant negative cdc2 mutant had little effect on ER activity (not shown). These results strongly argue that the observed decrease of ER transcriptional activity by p27 is due to inactivation of cdk2 and further suggests the importance of cyclin A/cdk2 enzymatic activity for hormone-dependent transcriptional enhancement by ER. It appears then, that the balance among the cdk regulatory proteins, cyclins, CAK, and CDIs, is critical in determining ER transcriptional activity.
Phosphorylation of ER by the Cyclin A/cdk2 Complex.
Next, we investigated whether the cyclin A/cdk2 complex can phosphorylate ER. To determine if ectopic expression of cyclin A increased the amount of phosphate incorporated into ER in vivo, HeLa cells were transfected with ER alone or in combination with cyclin A and cells were metabolically labeled with [32P]orthophosphate for 2 h in the presence or absence of 17β-estradiol. For each sample, the total amount of ER visualized by silver staining was used to standardize the amount of incorporated 32P. The untreated ER condition was arbitrarily set as 1. As shown in Fig. 3A, ER phosphorylation is increased by ectopic expression of cyclin A in both the absence (3×) and presence (3.7×) of hormone. Thus, the presence of cyclin A increases incorporation of phosphate into ER by activating endogenous cdks.
Figure 3.
ER phosphorylation by cyclin A/cdk2. (A) Phosphorylation of ER in vivo in the absence and presence of cyclin A. HeLa cells were transfected with FLAG-ER in the absence and presence of cyclin A expression vector using the Lipofectamine method as described in Fig. 1 and metabolically labeled with [32P]orthophosphate as described in Materials and Methods. Whole cell extracts were prepared and ER was immunoprecipitated using the FLAG-mAb, M2. ER immunoprecipitates were separated by SDS/10% polyacrylamide gel, silver-stained (Lower), and exposed to film to visualize the phosphorylated receptor (Upper). The incorporated radioactivity was normalized to the amount of ER immunoprecipitated in each condition. The value of the untreated ER was arbitrarily set as 1. (B) Phosphorylation of ER in vitro by the cyclin A/cdk2 complex. Bacterially expressed GST ER 1–82, 1–115, and 1–121 derivatives were absorbed onto glutathione agarose beads and used as substrates for in vitro kinase assays. Cyclin A and cdk2 were produced in 5B insect cells, separately or in combination, purified by immunoprecipitation, and used in the kinase assays as described in Materials and Methods. The proteins were separated by SDS/10% polyacrylamide gel and the phosphorylated products were visualized by autoradiography.
To further investigate the effect of cyclin A/cdk2-dependent phosphorylation of ER we performed in vitro kinase assays. Three ER derivatives, containing amino acids 1–82, 1–115 or 1–121 were bacterially expressed and purified as GST-fusion proteins and used as substrates for phosphorylation by immunopurified baculovirus-expressed cyclin A and cdk2. These particular derivatives were chosen since the ER 1–121 derivative contains three serine-proline motifs at Ser-104, -106, and -118, whereas ER 1–115 contains only Ser-104 and -106. ER 1–82 lacks all of the putative serine-proline phosphorylation sites and thus serves as a negative control. Fig. 3B demonstrates that both ER 1–121 and ER 1–115 were phosphorylated by the cyclin A/cdk2 complex but not by either subunit alone. On the other hand, ER 1–82 was not phosphorylated by the cyclin A/cdk2 complex. In each reaction, expression of the ER substrate and the kinase subunits was verified by Western blotting and found to be identical (not shown). The fact that ER 1–121 and ER 1–115 derivatives were phosphorylated while ER 1–82 was not, strongly suggests that the residues contained in the region comprised by amino acids 83–121 comprise a motif targeted by the cyclin A/cdk2 complex. This data provide in vitro biochemical evidence that ER is a substrate for cyclin A/cdk2-dependent phosphorylation.
Increased ER Transcriptional Enhancement in Response to Ectopic Cyclin A Expression in Multiple Cell Lines.
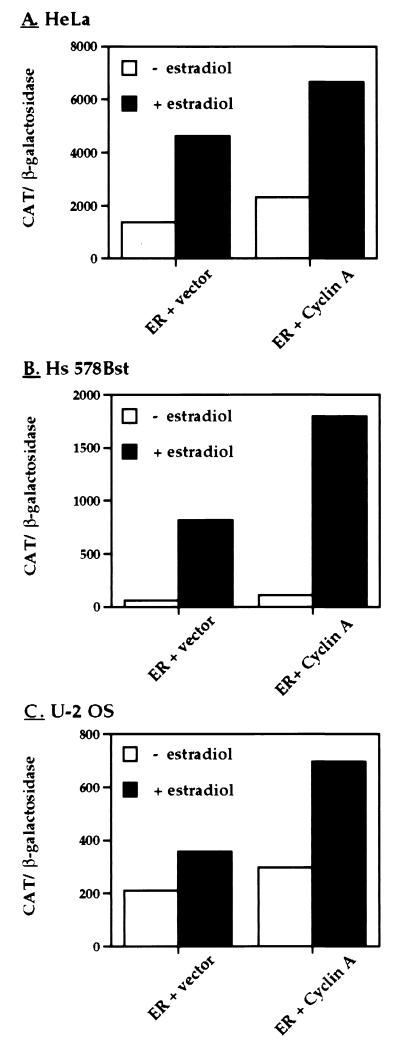
To test our hypothesis that the regulation of ER-dependent transcriptional activity by the cyclin A/cdk2 complex is not specific to HeLa cells but rather reflects a general mode of regulation, we repeated our transcriptional activity assay in a variety of cell lines. We tested Hs 578Bst cells derived from breast tissue peripheral to an infiltrating ductal carcinoma and an ER-negative osteosarcoma cell line, U-2 OS. Cells were transiently transfected as described above, treated with 17β-estradiol or the ethanol vehicle for 24 h and transcriptional activity was measured. In the three cell types utilized, we observed a significant increase in ER ligand-dependent and -independent transcriptional activation (Fig. 4). These data imply that the ability of a cyclin A/cdk2 complex to enhance ER ligand-dependent transcription is conserved across multiple cell types.
Figure 4.
Cyclin A enhances ER-dependent transcriptional activation in multiple human cell lines. Three ER-negative cell lines, (A) HeLa cells derived from a human cervical carcinoma, (B) Hs 578Bst, a human cell line derived from normal breast tissue, and (C) U-2 OS, a human osteosarcoma cell line, were transfected with ER expression and reporter plasmids as described in Fig. 1. Cells were also cotransfected with the empty expression vector or the expression vector encoding cyclin A. Hormone treatment and activity assays were performed as described in Fig. 1. Results shown represent a single experiment done in duplicate whose error was <10%. This experiment was repeated twice more with similar results.
DISCUSSION
We have examined the effects of cyclin A/cdk2 activation and inhibition on ER-dependent transcriptional enhancement. Here, we provide evidence that alterations in the regulation of the cyclin A/cdk2 complex lead to changes in both hormone-independent and hormone-dependent ER transcriptional enhancement. Our findings indicate that the ectopic expression of cyclin A elevates ER transcriptional activity and that this effect is not restricted to a single cell type. Our results likely represent an underestimate of the full impact of cyclin A on ER transcriptional activity, since these findings are obtained in cells that contain endogenous cyclin A and cdk2. Consistent with this view, ER transcriptional activity in both the presence and absence of hormone is virtually abolished under conditions where cyclin A/cdk2 activity is suppressed by the kinase inhibitor p27, or by a dominant negative cdk2 mutant. Thus it appears that cyclin A, the regulatory subunit of cdk2, is a limiting cofactor in the regulation of ER-dependent transcriptional activation in the cells examined.
Our findings also demonstrate that ER is a substrate for cyclin A/cdk2. ER is phosphorylated in vitro by cyclin A/cdk2 complexes and incorporation of phosphate into ER is stimulated by cyclin A expression in vivo. Importantly, our biochemical data demonstrate that the presence of three putative cdk target sites (Ser-104, Ser-106, Ser-118) is correlated with cyclin A/cdk2-dependent phosphorylation of the ER substrate in vitro. Work is ongoing to identify the precise residue(s) on the receptor phosphorylated by the cyclin A/cdk2 complex. Together, these data suggest that the cyclin A/cdk2 complex directly phosphorylates ER and that this modification serves to increase the receptor’s transcriptional regulatory properties.
Zwijsen et al. (4) observed that overexpression of cyclin D1 increased ER-dependent transcriptional activation in T-47D cells without direct ER phosphorylation by cyclin D1/cdk4. Cyclin D1 appears to act indirectly as an ER cofactor, perhaps by tethering or phosphorylating other regulatory proteins that effect downstream signaling by ER. In contrast, we observed the cyclin A/cdk2 complex directly acting upon the receptor leading to its phosphorylation and a marked increase in ER-dependent transcriptional activity. Zwijsen et al. (4) did not observe an effect of cyclin A overexpression on ER-dependent transcriptional activation in T-47D cells and conversely, we failed to detect an increase in ER activity when cyclin D1 was ectopically expressed in either HeLa or ER-expressing U-2 OS human osteosarcoma cell line (J.M.T. and M.J.G., unpublished data). One factor contributing to the observed differences may lie in the cell types used in these studies. Recent reports and our own observations suggest that the level of expression of CDIs, such as p27, differ dramatically among cell types (34). Given that these proteins function as kinase inhibitors and as recently recognized cyclin D/cdk4 assembly factors, differences in CDI expression might significantly alter cdk signaling (34, 35). Among several breast cancer cell lines tested, T-47D cells were found to express high levels of p27 (34). This finding may account for the lack of a cyclin A effect in these cells, since the resulting cyclin A/cdk2 complex will be inhibited by endogenous p27. In contrast, HeLa cells used in this study express comparatively low amounts of p27, making ER-dependent transcription more sensitive to ectopic cyclin A expression. The cell-specific differences in the level of endogenous p27 may also help explain the ability of cyclin D1 to activate ER in T-47D cells, but not in HeLa cells, since abundant p27 may favor the formation of a cyclin D1/cdk4 complex, which may in turn phosphorylate an ER coactivator, or facilitate complex formation between ER and a receptor cofactor. Examination of the consequences of ectopic cyclin A expression in several breast cell lines has revealed an inverse correlation between cyclin A activation of ER transcriptional enhancement and the level of endogenous p27 (J.M.T. and M.J.G., unpublished data). Thus, the level of endogenous CDI may determine which cyclin isotypes will affect ER transcriptional activity, and may account for the observed differences between our findings and that of the Zwijsen et al (4).
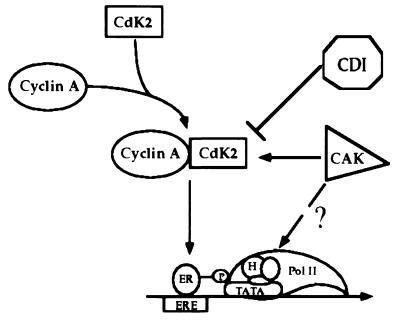
Based on our findings, we propose a model for ER regulation by the cyclin A/cdk2 complex (Fig. 5). The cyclin A/cdk2 complex directly phosphorylates the receptor and in doing so, facilitates its interaction with the basal transcriptional machinery or an ER coactivator, which increases the receptor’s ability to activate transcription. Inhibition of cdk activity by CDIs, such as p27, or through a reduction in cyclin or cdk expression, would decrease receptor phosphorylation, weakening these putative ER-transcription factor contacts, thus leading to decreased receptor transcriptional activity. We further envision that the expression of the CAK complex, cyclin H and cdk7, enhances ER transcriptional activation by increasing the activity of the endogenous cyclin A/cdk2 pool. Since cyclin H and cdk7 are also components of TFIIH (5, 36–38), we cannot exclude the possibility that this complex may be acting at the level of TFIIH to increase its catalytic activity, which in turn, increases ER transcriptional activity. Together, these data suggest that the cyclin A/cdk2 complex directly influences ER’s transcriptional regulatory properties. We conclude that ultimately the balance of these cdk regulatory proteins determines kinase activity, which in this case translates into differential transcriptional activation by ER.
Figure 5.
Model for regulation of ER-dependent transcriptional activation by the cyclin A/cdk2 complex. The cyclin A/cdk2 complex phosphorylates ER which increases the receptor’s ability to activate transcription by facilitating its interaction with the basal transcriptional machinery or an ER coactivator. Inhibiting cdk activity by CDIs has the opposite effect, resulting in reduced ER phosphorylation and decreased receptor transcriptional activity. Expression of the CAK complex, cyclin H, and cdk7, enhances ER-dependent transcription by increasing the activity of the endogenous cyclin A/cdk2 pool. It is also conceivable that the CAK complex may be acting at the level of TFIIH to increase ER transcriptional enhancement. We conclude that it is the balance among the cyclins, cdks, and their regulatory proteins that will ultimately determine ER transcriptional activity.
A complex picture of signal transduction by ER is emerging that appears to rely on the collaboration of multiple factors for its regulation, with each event in the pathway vulnerable to subversion. This subversion may take the form of aberrant expression of cyclin or cdk subunits, or CDIs, leading to alterations in receptor phosphorylation and activity that might contribute to uncontrolled cell proliferation. Clearly, the involvement of cyclins, cdks, CAKs, and CDIs in ER-mediated transcriptional regulation is complex and will require further investigation. It is likely, that phosphorylation events mediated by the cyclin/cdk pathway will emerge as a general mechanism of controlling steroid hormone action (31, 39).
Acknowledgments
We thank N. Tanese and members of the Garabedian laboratory for critically reading the manuscript. We thank D. Morgan (University of California, San Francisco) for generously supplying the cDNAs and baculovirus strains encoding cyclin A, cdk2, cyclin H, and cdk7, and J. Massagué (Memorial Sloan–Kettering) for the p27 expression construct. This work was supported by grants to M.J.G. from the Army Breast Cancer Research Fund (DAMD17-94-J-4454 and DAMD17-96-1-6032), the Whitehead Fellowship for Junior Faculty in Biological Sciences, and the Kaplan Cancer Center.
ABBREVIATIONS
- ER
estrogen receptor
- cdk
cyclin-dependent kinase
- CAK
cyclin-dependent kinase activator
- CDI
cyclin-dependent kinase inhibitor
- CAT
chloramphenicol acetyltransferase
- β-gal
β-galactosidase
- GST
glutathione S-transferase
References
- 1.Le Goff P, Montano M M, Schodin D J, Katzenellenbogen B S. J Biol Chem. 1994;269:4458–4466. [PubMed] [Google Scholar]
- 2.Katzenellenbogen B S, Bhardwaj B, Fang H, Ince B A, Pakdel F, Reese J, Schodin D, Wrenn C K. J Steroid Biochem Mol Biol. 1993;47:39–48. doi: 10.1016/0960-0760(93)90055-2. [DOI] [PubMed] [Google Scholar]
- 3.Ali S, Metzger D, Bornert J-M, Chambon P. EMBO J. 1993;12:1153–1160. doi: 10.1002/j.1460-2075.1993.tb05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwijsen R M, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R J A M. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 5.Fisher R P. Curr Opin Genet Dev. 1997;7:32–38. doi: 10.1016/s0959-437x(97)80106-2. [DOI] [PubMed] [Google Scholar]
- 6.Elledge S J, Harper J W. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 7.Mathias P, Herskowitz I. Cell. 1994;79:181–184. doi: 10.1016/0092-8674(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 8.Morgan D O. Nature (London) 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 9.Keyomarsi K, Pardee A B. Proc Natl Acad Sci USA. 1993;90:1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartkova J, Lukas J, Muller H, Strauss M, Gusterson B, Bartek J. Cancer Res. 1995;55:949–956. [PubMed] [Google Scholar]
- 11.Buckley M F, Sweeney K J, Hamilton J A, Sini R L, Manning D L, Nicholson R I, deFazio A, Watts C K, Musgrove E A, Sutherland R L. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 12.Cordon-Cardo C. Am J Pathol. 1995;147:545–560. [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta A, Chandra R, Leiter L M, Lester S. Proc Natl Acad Sci USA. 1995;92:5386–5390. doi: 10.1073/pnas.92.12.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong J, Ardelt B, Traganos F, Darzynkiewicz Z. Cancer Res. 1994;54:4285–4288. [PubMed] [Google Scholar]
- 15.Loda M, Cukor B, Tam S W, Lavin P, Fiorentino M, Draetta G F, Jessup J M, Pagano M. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 16.Gray-Bablin J, Zalvide J, Fox M P, Knickerbocker C J, DeCaprio J A, Keyomarsi K. Proc Natl Acad Sci USA. 1996;93:15215–15220. doi: 10.1073/pnas.93.26.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catzavelos C, Bhattacharya N, Ung Y C, Wilson J A, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard K, Slingerland J. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 18.Tan P, Cady B, Wanner M, Worland P, Cukor B, Magi-Galluzi C, Lavin P, Draetta G, Pagano M, Loda M. Cancer Res. 1997;57:1259–1263. [PubMed] [Google Scholar]
- 19.Guadagno T M, Ohtsubo M, Roberts J M, Assoian R K. Science. 1993;262:1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- 20.Barrett J F, Lewis B C, Hoang A T, Alvarez R J, Jr, Dang C V. J Biol Chem. 1995;270:15923–15925. doi: 10.1074/jbc.270.27.15923. [DOI] [PubMed] [Google Scholar]
- 21.Henglein B, Chenivesse X, Wang J, Eick D, Brechot C. Proc Natl Acad Sci USA. 1994;91:5490–5494. doi: 10.1073/pnas.91.12.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg A R, Zindy F, Le Deist F, Mouly H, Metezeau P, Brechot C, Lamas E. Oncogene. 1995;10:1501–1509. [PubMed] [Google Scholar]
- 23.Said T K, Medina D. Carcinogenesis. 1995;16:823–830. doi: 10.1093/carcin/16.4.823. [DOI] [PubMed] [Google Scholar]
- 24.Brechot C. Curr Opin Gen Dev. 1993;3:11–18. doi: 10.1016/s0959-437x(05)80335-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Nature (London) 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 26.Resnitzky D, Hengst L, Reed S I. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall F L, Williams R T, Wu L, Wu F, Carbonaro-Hall D A, Harper J W, Warburton D. Oncogene. 1993;8:1377–1384. [PubMed] [Google Scholar]
- 28.Bremner R, Cohen B L, Sopta M, Hamel P A, Ingles C J, Gallie B L, Phillips R A. Mol Cell Biol. 1995;15:3256–3265. doi: 10.1128/mcb.15.6.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. In: Current Protocols in Molecular Biology. Ausubel F, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Vol. 1. New York: Wiley; 1996. pp. 9.1.4–9.1.11. [Google Scholar]
- 30.Sleigh M J. Anal Biochem. 1986;156:251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- 31.Krstic M D, Rogatsky I, Yamamoto K R, Garabedian M J. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polyak K, Lee M H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massague J. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 33.Toyoshima H, Hunter T. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 34.Fredersdorf S, Burns J, Milne A M, Packham G, Fallis L, Gillet C E, Royds J A, Peston D, Hall P A, Hanby A M, Barnes D M, Shousha S, O’Hare M J, Lu X. Proc Natl Acad Sci USA. 1997;94:6380–6385. doi: 10.1073/pnas.94.12.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 36.Adamczewski J P, Rossignol M, Tassan J P, Nigg E A, Moncollin V, Egly J M. EMBO J. 1996;15:1877–1884. [PMC free article] [PubMed] [Google Scholar]
- 37.Drapkin R, Le Roy G, Cho H, Akoulitchev S, Reinberg D. Proc Natl Acad Sci USA. 1996;93:6488–6493. doi: 10.1073/pnas.93.13.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiekhattar R, Mermelstein F, Fisher R P, Drapkin R, Dynlacht B, Wessling H C, Morgan D O, Reinberg D. Nature (London) 1995;374:283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Beck C A, Weigel N L. Mol Endocrinol. 1997;11:825–833. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]