Abstract
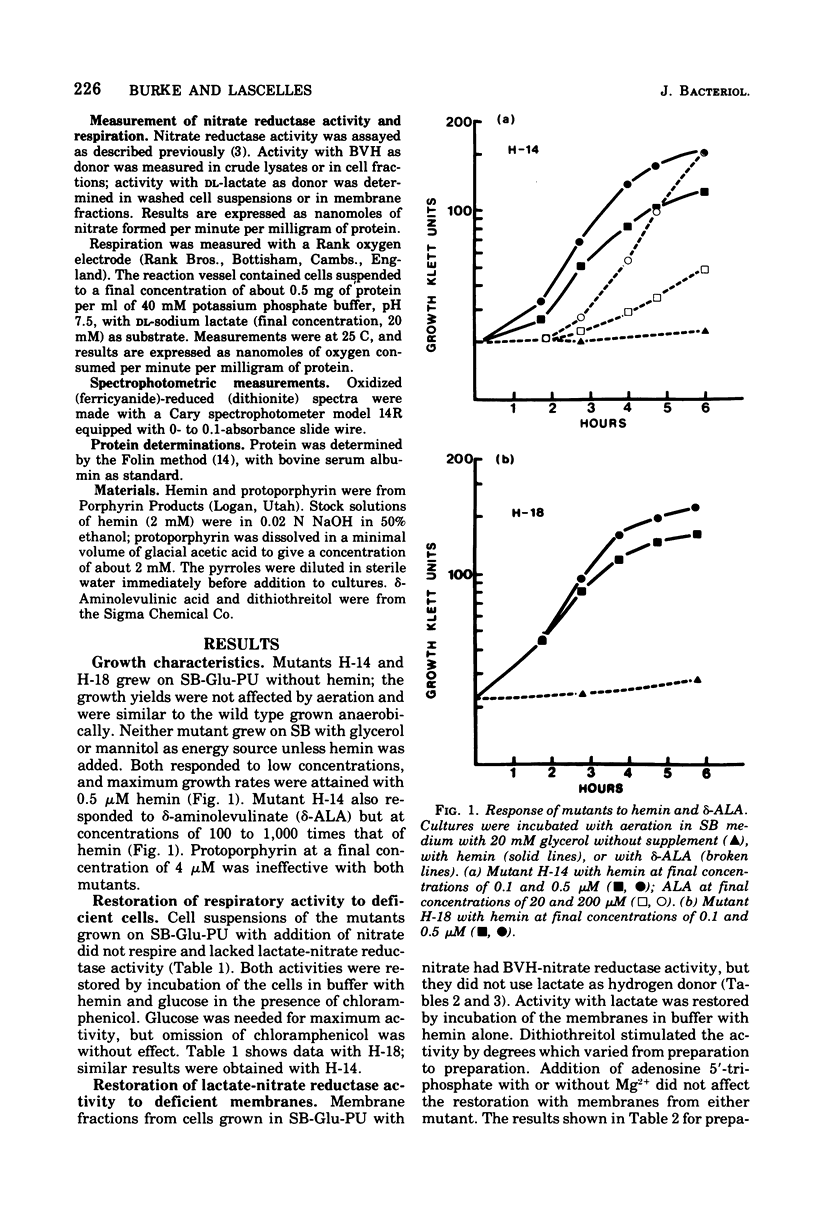
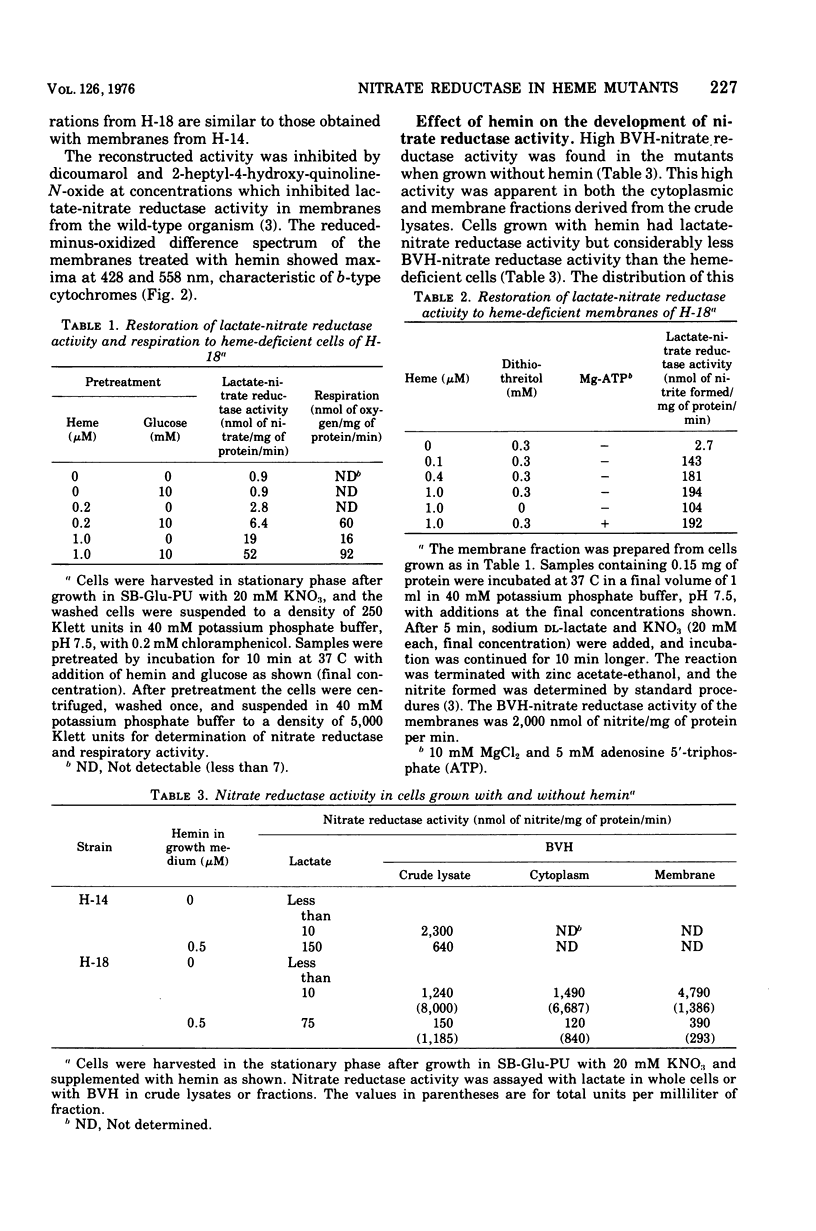
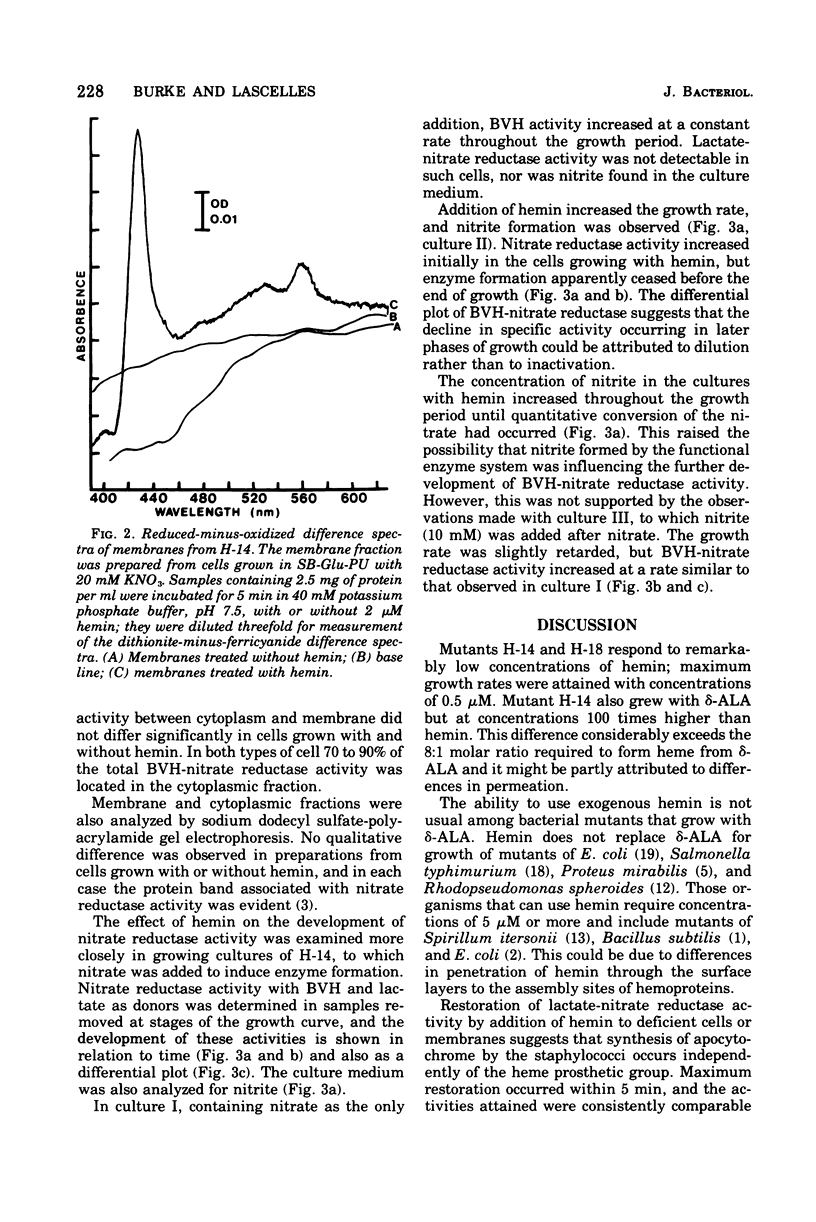
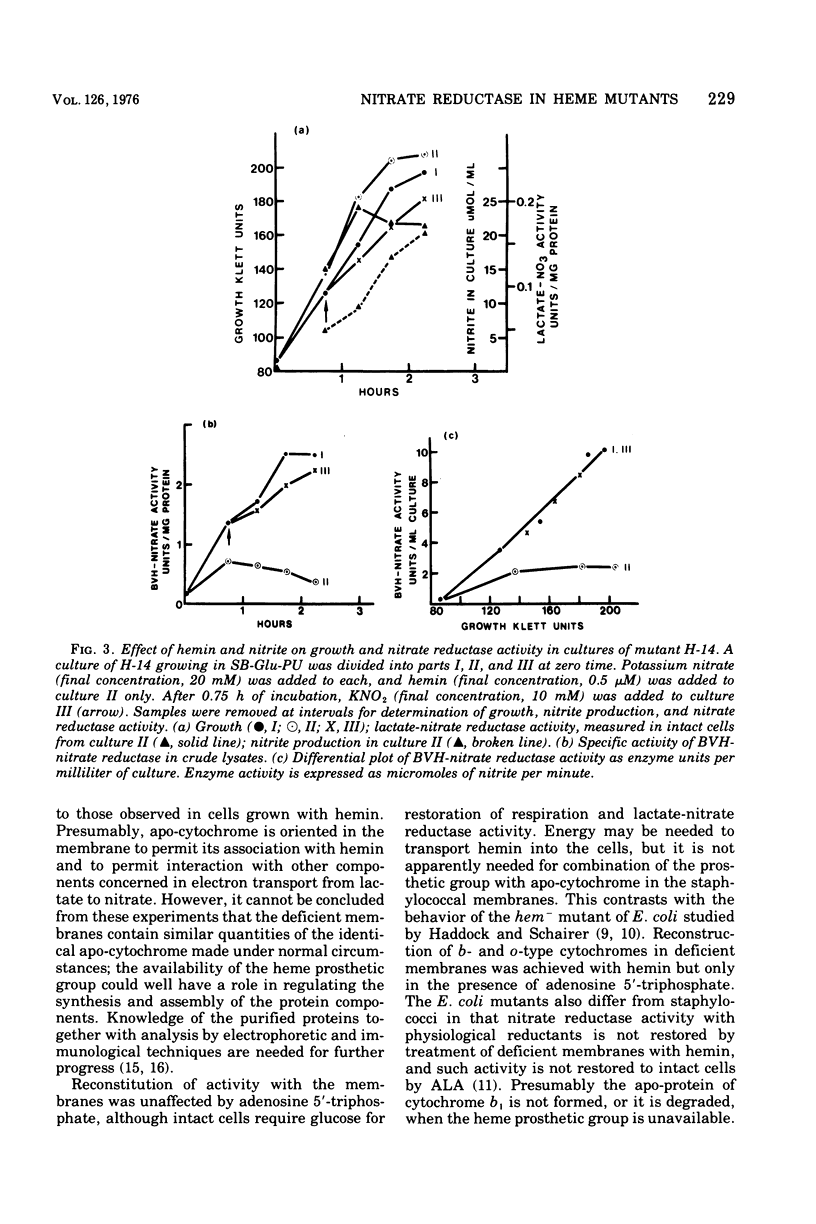
Mutants H-14 and H-18 of Staphylococcus aureus require hemin for growth on glycerol and other nonfermentable substrates. H-14 also responds to delta-aminolevulinate. Heme-deficient cells grown in the presence of nitrate do not have lactate-nitrate reductase activity but gain this activity when incubated with hemin in buffer and glucose. Lactate-nitrate reductase activity is also restored to the membrane fraction from such cells by incubation with hemin and dithiothreitol; addition of adenosine 5'-triphosphate has no effect upon the restoration. Cells grown with nitrate in the absence of hemin have two to five times more reduced benzyl viologen-nitrate reductase activity than do those grown with hemin. The activity increases throughout the growth period in the absence of hemin, but with hemin present enzyme formation ceases before the end of growth. There was no evidence of enzyme destruction. The distribution of nitrate reductase activity between membrane and cytoplasm was similar in cells grown with and without hemin; 70 to 90% was in the cytoplasm. It is concluded that heme-deficient staphylococci form apo-cytochrome b, which readily combines in vitro with its prosthetic group to restore normal function. The avaliability of the heme prosthetic group influences the formation of nitrate reductase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. J., Ivánovics G. Isolation and some characteristics of haemin dependent mutants of Bacillus subtilis. J Gen Microbiol. 1967 Oct;49(1):31–40. doi: 10.1099/00221287-49-1-31. [DOI] [PubMed] [Google Scholar]
- BELJANSKI M. Sur la formation d'enzymes respiratoires chez un mutant d'Escherichia coli streptomycino-résistant et auxotrophe pour l'hémine. Ann Inst Pasteur (Paris) 1957 Mar;92(3):396–412. [PubMed] [Google Scholar]
- Burke K. A., Lascelles J. Nitrate reductase system in Staphylococcus aureus wild type and mutants. J Bacteriol. 1975 Jul;123(1):308–316. doi: 10.1128/jb.123.1.308-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG J. P., LASCELLES J. NITRATE REDUCTASE IN CELL-FREE EXTRACTS OF A HAEMIN-REQUIRING STRAIN OF STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Dec;89:503–510. doi: 10.1042/bj0890503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot G. N., Stouthamer A. H. Regulation of reductase formation in Proteus mirabilis. II. Influence of growth with azide and of haem deficiency on nitrate reductase formation. Biochim Biophys Acta. 1970 Jun;208(3):414–427. doi: 10.1016/0304-4165(70)90214-x. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The role of a novel cytochrome b-containing nitrate reductase and quinone in the in vitro reconstruction of formate-nitrate reductase activity of E. coli. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1234–1241. doi: 10.1016/s0006-291x(74)80416-x. [DOI] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Pleiotropic menaquinone-deficient mutant of Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1021–1034. doi: 10.1128/jb.115.3.1021-1034.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenbaum P. E., Keyser P. D., White D. C. Role of vitamin K2 in the organization and function of Staphylococcus aureua membranes. J Bacteriol. 1975 Feb;121(2):442–449. doi: 10.1128/jb.121.2.442-449.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A. The reconstitution of oxidase activity in membranes derived from a 5-aminolaevulinic acid-requiring mutant of Escherichia coli. Biochem J. 1973 Dec;136(4):877–884. doi: 10.1042/bj1360877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J., Altschuler T. Mutant strains of Rhodopseudomonas spheroides lacking delta-aminolevulinate synthase: growth, heme, and bacteriochlorophyll synthesis. J Bacteriol. 1969 May;98(2):721–727. doi: 10.1128/jb.98.2.721-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J., Rittenberg B., Clark-Walker G. D. Growth and cytochrome synthesis in a hemin-requiring mutant of Spirillum itersonii. J Bacteriol. 1969 Jan;97(1):455–456. doi: 10.1128/jb.97.1.455-456.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Anaerobic cytochrome b1 in Escherichia coli: association with and regulation of nitrate reductase. J Bacteriol. 1975 Mar;121(3):1111–1116. doi: 10.1128/jb.121.3.1111-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H. Synthesis of nitrate reductase components in chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1975 Mar;121(3):1117–1121. doi: 10.1128/jb.121.3.1117-1121.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synthesis and sideedness of membrane-bound respiratory nitrate reductase (EC1.7.99.4) in Escherichia coli lacking cytochromes. Biochem J. 1975 May;148(2):329–333. [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Sanderson K. E., Surdeanu M., Sonea S. Hemin-deficient mutants of Salmonella typhimurium. J Bacteriol. 1970 May;102(2):531–536. doi: 10.1128/jb.102.2.531-536.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien W., White D. C. Linear sequential arrangement of genes for the biosynthetic pathway of protoheme in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1392–1398. doi: 10.1073/pnas.61.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]



