Abstract
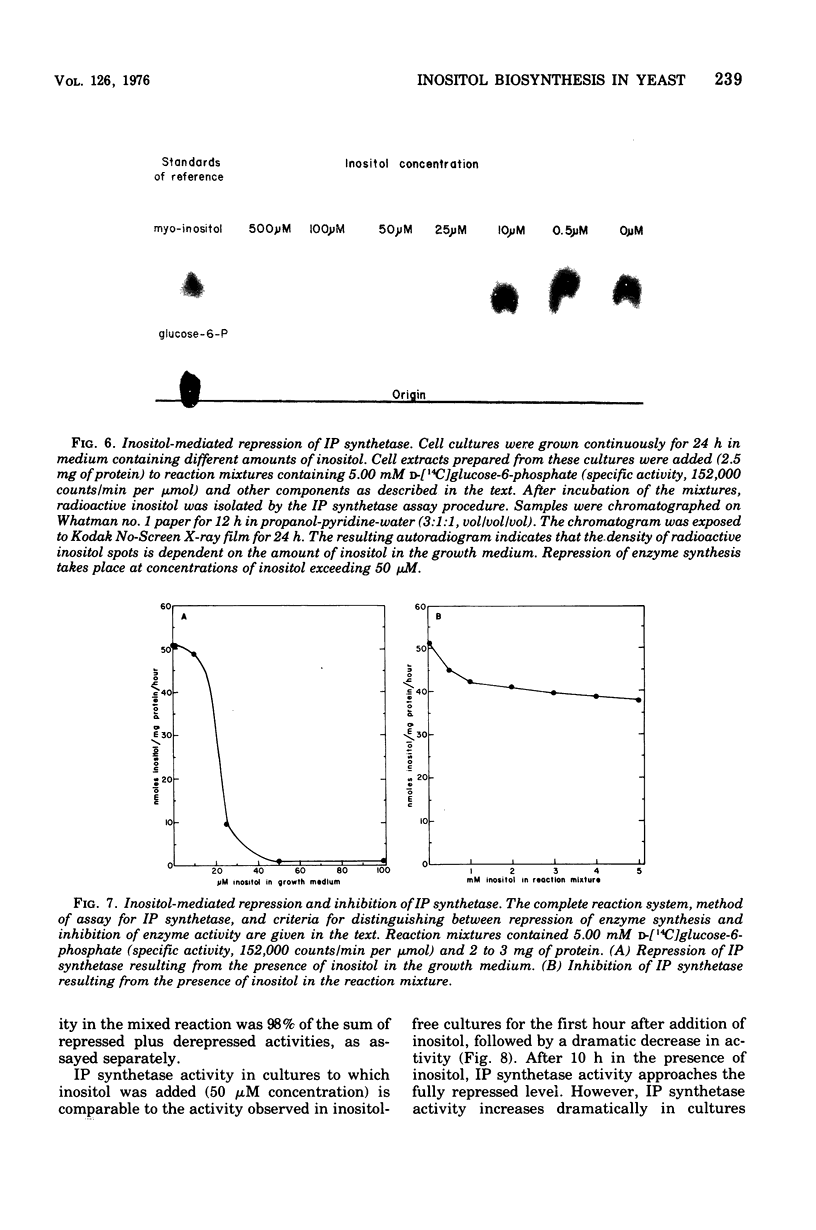
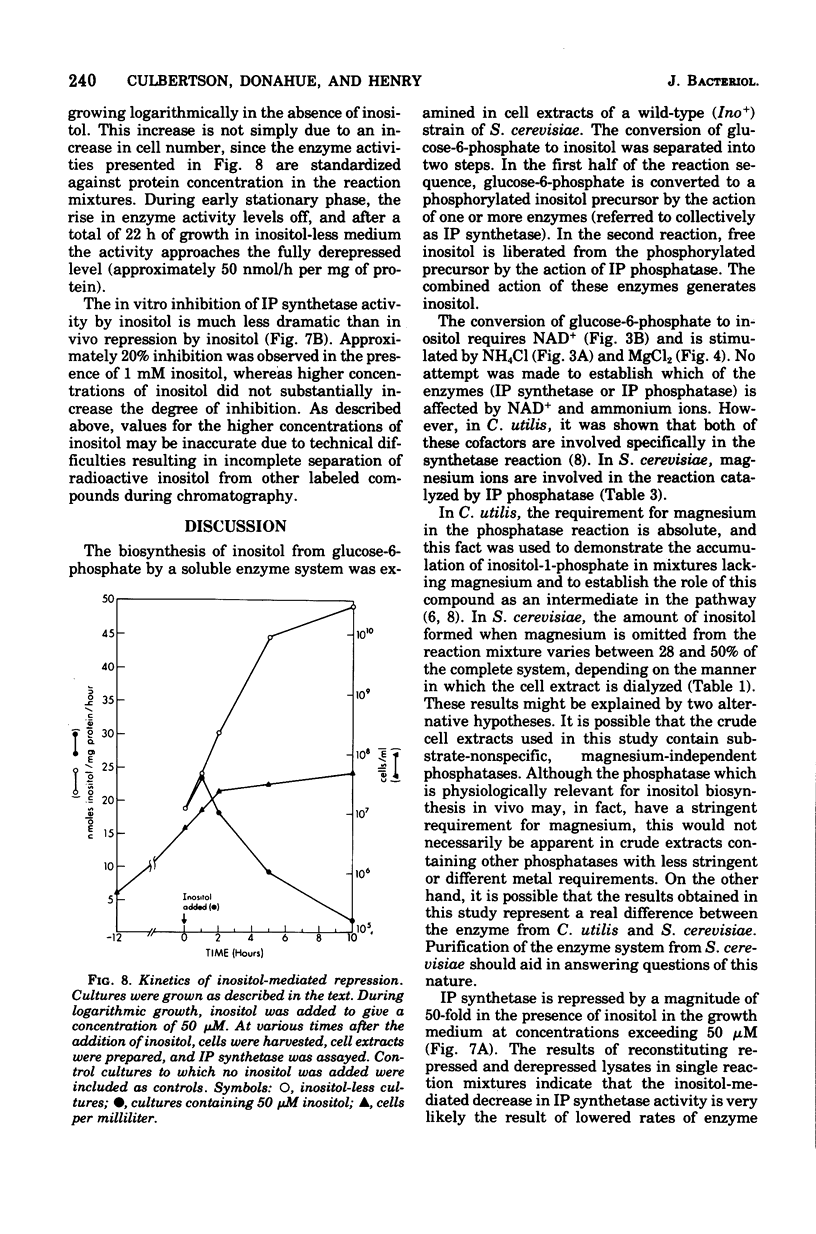
Inositol biosynthesis was studied in soluble, cell extracts of a wild-type (Ino) strain of Saccharomyces cerevisiae. Two reactions were detected: (i) conversion of D-glucose-6-phosphate to a phosphorylated form of inositol, presumably inositol-1-phosphate (IP synthethase, EC5.5.1.4), and (ii) conversion of phosphorylated inositol to inositol (IP phosphatase, EC3.1.3.25). The in vitro rate of conversion of glucose-6-phosphate to inositol was proportional to incubaion time and enzyme concentration. The pH optimum was 7.0. The synthesis of inositol required oxidized nicotinamide adenine dinucleotide (NAD) and was stimulated byNH4C1 and MgC12. NADP substituted poorly for NAD, and NADH inhibitedthe reaction. Phosphorylated inositol accumulated in the absence of MgC12, suggesting that inositol-phosphate is an intermediate in the pathway and that Mg ions stimulate the dephosphorylation of inositol-phosphate. IP synthetase was inhibited approximately 20% in the presence of inositol in the reaction mixture at concentrations exceeding 1 mM. The enzyme was repressed approximately 50-fold when inositol was present in the growth medium at concentrations exceeding 50 muM. IP synthetase reached the fully repressed level approximately 10 h after the addition of inositol to logarithmic cultures grown in the absence of inositol. The specific activity of the enzyme increased with time in logarithmically growing cultures lacking inositol andapproached the fully depressed level as the cells entered stationary phase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus W. W., Lester R. L. Turnover of inositol and phosphorus containing lipids in Saccharomyces cerevisiae; extracellular accumulation of glycerophosphorylinositol derived from phosphatidylinositol. Arch Biochem Biophys. 1972 Aug;151(2):483–495. doi: 10.1016/0003-9861(72)90525-5. [DOI] [PubMed] [Google Scholar]
- Barnett J. E., Rasheed A., Corina D. L. Partial reactions of D-glucose 6-phosphate-1L-myoinositiol 1-phosphate cyclase. Biochem J. 1973 Jan;131(1):21–30. doi: 10.1042/bj1310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN I. W., CHARALAMPOUS F. C. BIOCHEMICAL STUDIES ON INOSITOL. VII. BIOSYNTHESIS OF INOSITOL BY A SOLUBLE ENZYME SYSTEM. J Biol Chem. 1964 Jun;239:1905–1910. [PubMed] [Google Scholar]
- Chen I. W., Charalampous C. F. Biochemical studies on inositol. IX. D-Inositol 1-phosphate as intermediate in the biosynthesis of inositol from glucose 6-phosphate, and characteristics of two reactions in this biosynthesis. J Biol Chem. 1966 May 25;241(10):2194–2199. [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae; inositol-phosphate synthetase mutants. J Bacteriol. 1976 Apr;126(1):243–250. doi: 10.1128/jb.126.1.243-250.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A. Death resulting from fatty acid starvation in yeast. J Bacteriol. 1973 Dec;116(3):1293–1303. doi: 10.1128/jb.116.3.1293-1303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Horowitz B. A new method for mutant selection in Saccharomyces cerevisiae. Genetics. 1975 Feb;79(2):175–186. doi: 10.1093/genetics/79.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- Marzluf G. A., Metzenberg R. L. Positive control by the cys-3 locus in regulation of sulfur metabolism in Neurospora. J Mol Biol. 1968 Apr 28;33(2):423–437. doi: 10.1016/0022-2836(68)90199-x. [DOI] [PubMed] [Google Scholar]
- Pateman J. A., Cove D. J. Regulation of nitrate reduction in Aspergillus nidulans. Nature. 1967 Sep 16;215(5107):1234–1237. doi: 10.1038/2151234a0. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Stewart M. A., Zinbo M. Mass spectrometric study on the mechanism of D-glucose 6-phosphate-L-myo-inositol 1-phosphate cyclase. J Biol Chem. 1969 Oct 25;244(20):5703–5708. [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Metabolism of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Jan 27;260(1):82–87. doi: 10.1016/0005-2760(72)90076-8. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S., Smith S., Waechter C. J., Lester R. L. Isolation and partial characterization of a major inositol-containing lipid in baker's yeast, mannosyl-diinositol, diphosphoryl-ceramide. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1042–1048. doi: 10.1073/pnas.64.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]