Abstract
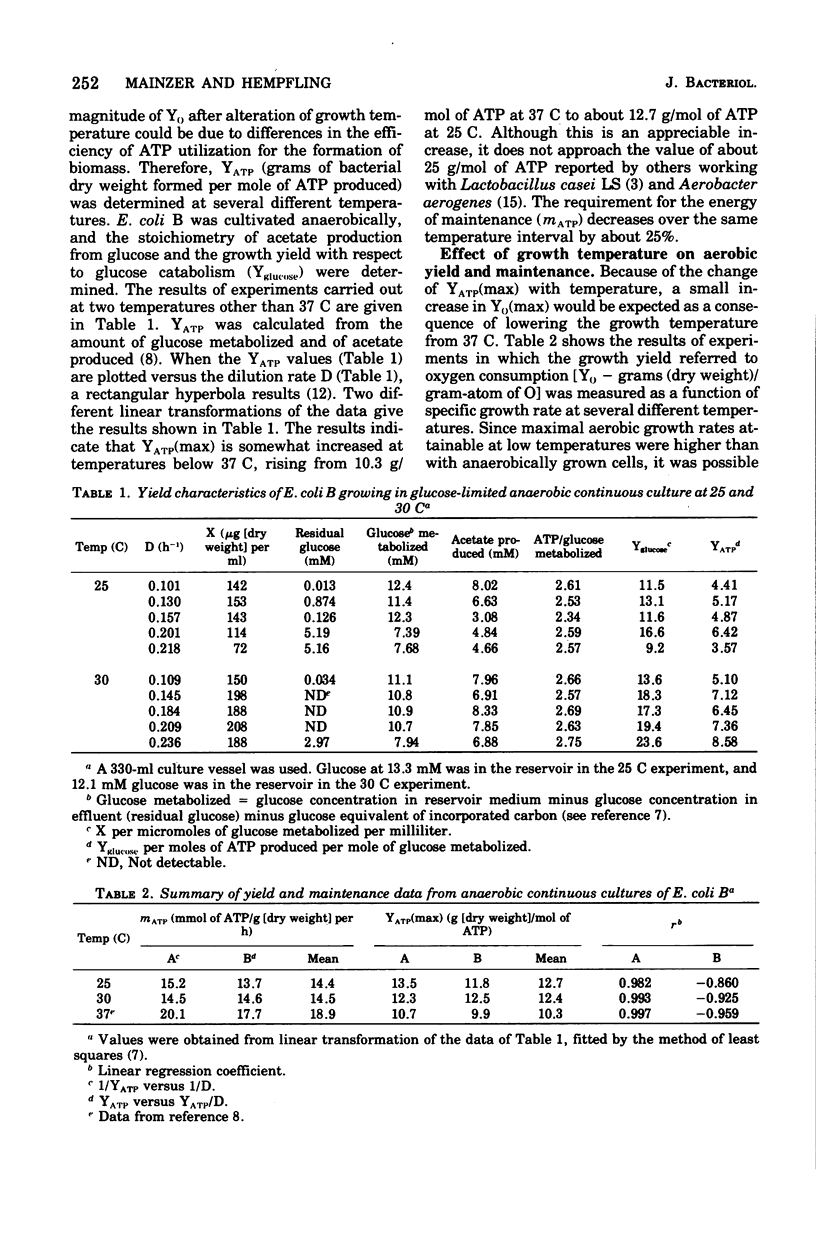
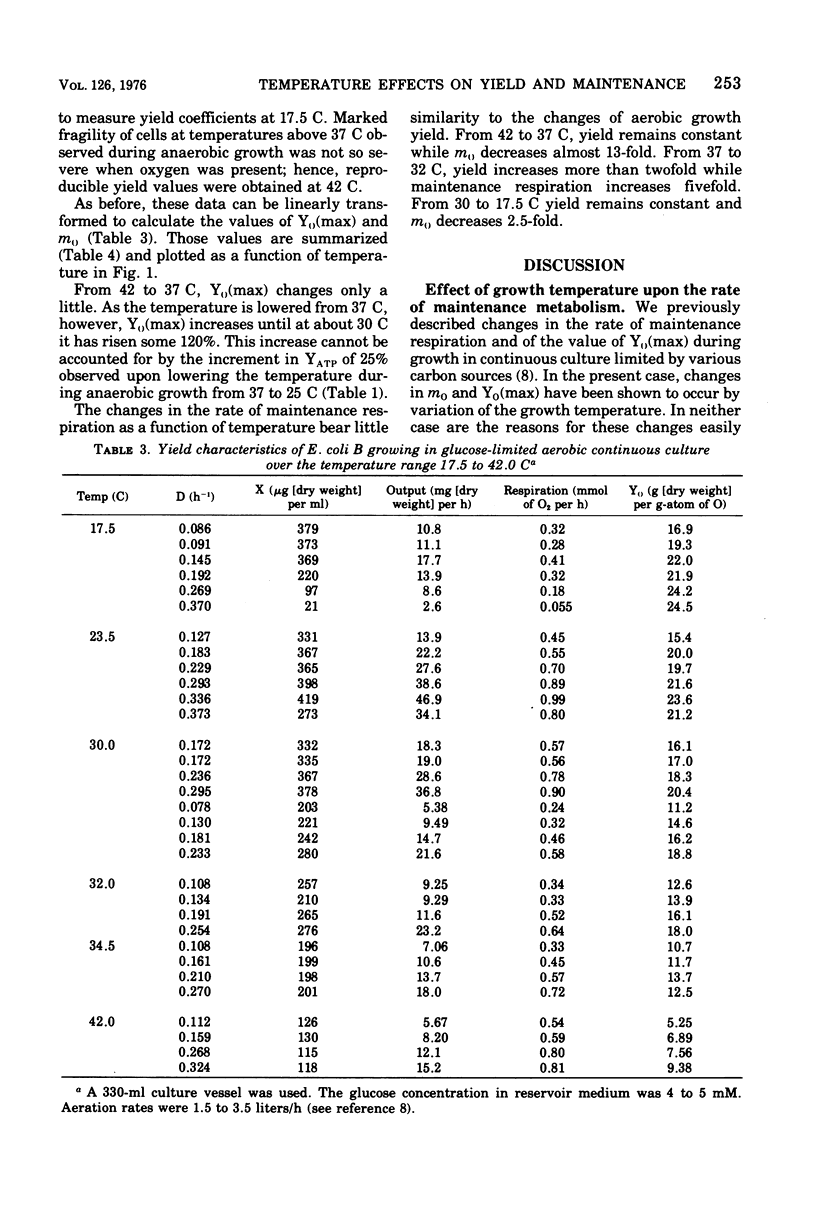
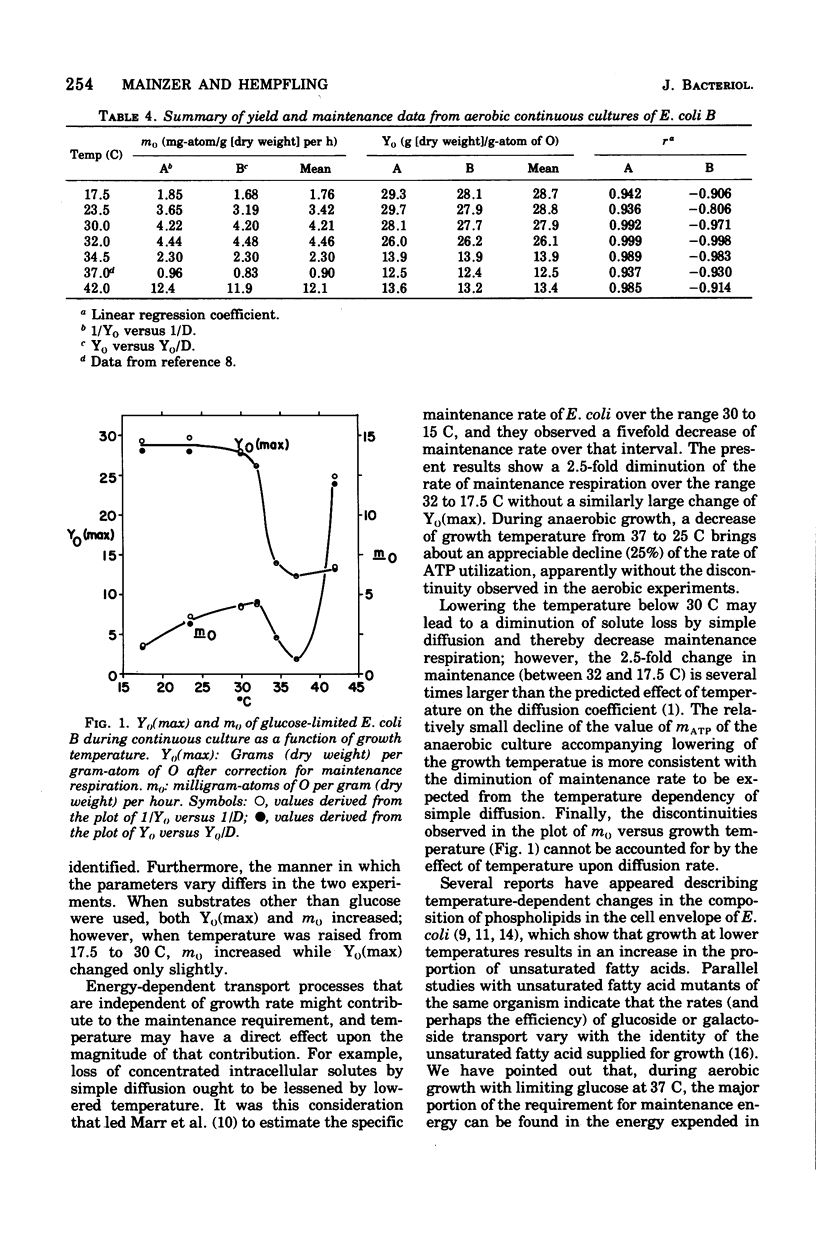
The effects of growth temperature on the aerobic growth yield with respect to oxygen consumption (Y0-grams [dry weight] per gram-atom of O) and the rate of maintenance respiration (m0-milligram-atoms of O/gram [dry weight] per hour) are reported for Escherichia coli B cultivated continuously in the presence of oxygen with limiting glucose. During anaerobic continuous culture, YATP(max) (grams [dry weight] per mole of ATP corrected for maintenance) increases from 10.3 to 12.7 as the growth temperature is lowered from 37 to 25 C. Over this same range, Y0(max) (Y0 corrected for maintenance respiration) rises from 12.5 to 28.8 and remains at the higher value down to 17.5 C. From 37 to 32 C, m0 increases from 0.9 to 4.4 but then falls to 1.5 as the temperature is lowered to 17.5 C. The value of m0 sharply rises some 13-fold as the temperature is raised to 42 C without a significant change in the value of Y0(max). Changes of Y0(max) are consistent with a temperature-sensitive doubling of the efficiency of oxidative phosphorylation, but the reasons for the changes of the rate of maintenance respiration are not known.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coultate T. P., Sundaram T. K. Energetics of Bacillus stearothermophilus growth: molar growth yield and temperature effects on growth efficiency. J Bacteriol. 1975 Jan;121(1):55–64. doi: 10.1128/jb.121.1.55-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Veldkamp H. A continuous culture study of an obligately psychrophilic Pseudomonas species. Arch Mikrobiol. 1967;59(1):123–130. doi: 10.1007/BF00406323. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Beeman D. K. Release of glucose repression of oxidative phosphorylation in Escherichia coli B by cyclic adenosine 3',5'-monophosphate. Biochem Biophys Res Commun. 1971 Nov;45(4):924–930. doi: 10.1016/0006-291x(71)90426-8. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Höfer M., Harris E. J., Pressman B. C. Correlation between changes in metabolite concentrations and rate of ion transport following glucose addition to Escherichia coli B. Biochim Biophys Acta. 1967 Jul 25;141(2):391–400. doi: 10.1016/0304-4165(67)90114-6. [DOI] [PubMed] [Google Scholar]
- Hempfling W. P., Mainzer S. E. Effects of varying the carbon source limiting growth on yield and maintenance characteristics of Escherichia coli in continuous culture. J Bacteriol. 1975 Sep;123(3):1076–1087. doi: 10.1128/jb.123.3.1076-1087.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P. Repression of oxidative phosphorylation in Escherichia coli B by growth in glucose and other carbohydrates. Biochem Biophys Res Commun. 1970 Oct 9;41(1):9–15. doi: 10.1016/0006-291x(70)90461-4. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H. Phospholipid metabolism in Escherichia coli after a shift in temperature. Biochim Biophys Acta. 1969 Jan 21;176(1):125–134. [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- SENEZ J. C. Some considerations on the energetics of bacterial growth. Bacteriol Rev. 1962 Jun;26:95–107. [PMC free article] [PubMed] [Google Scholar]
- Shaw M. K., Ingraham J. L. Fatty Acid Composition of Escherichia coli as a Possible Controlling Factor of the Minimal Growth Temperature. J Bacteriol. 1965 Jul;90(1):141–146. doi: 10.1128/jb.90.1.141-146.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]



