Abstract
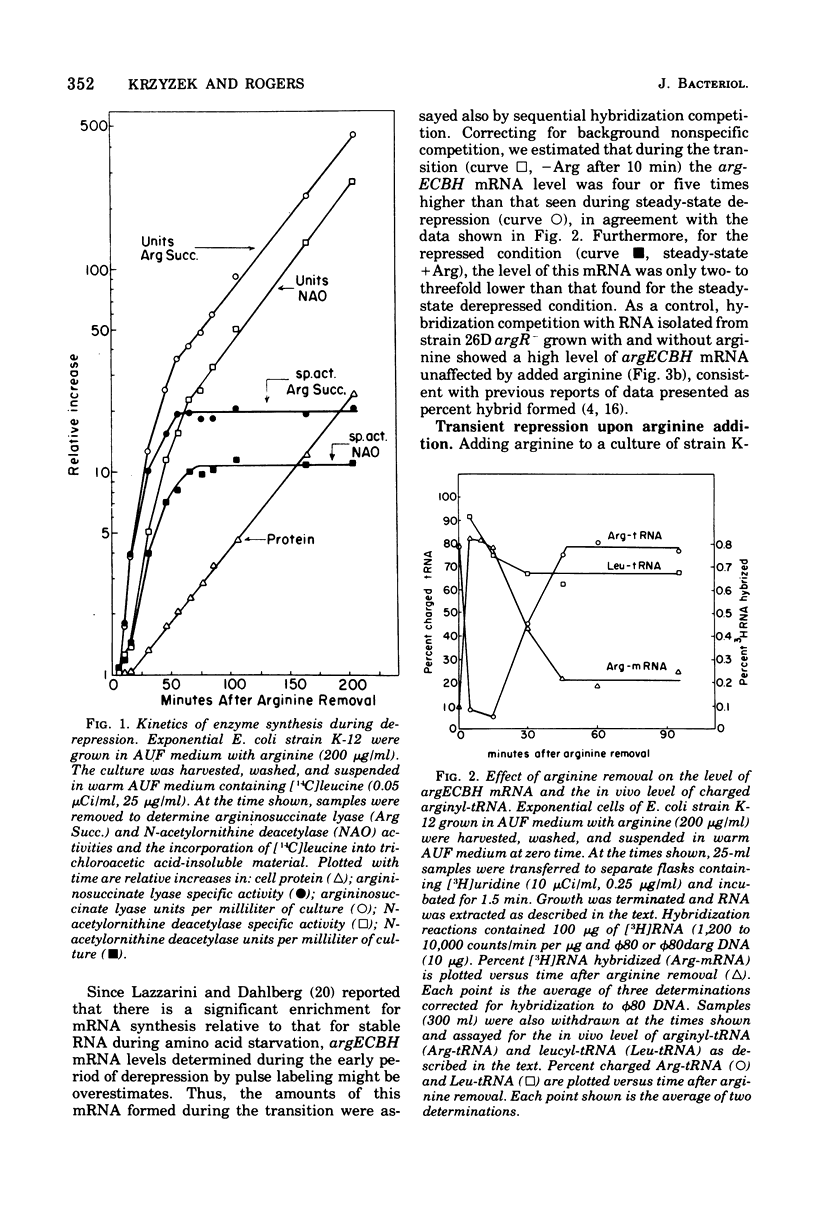
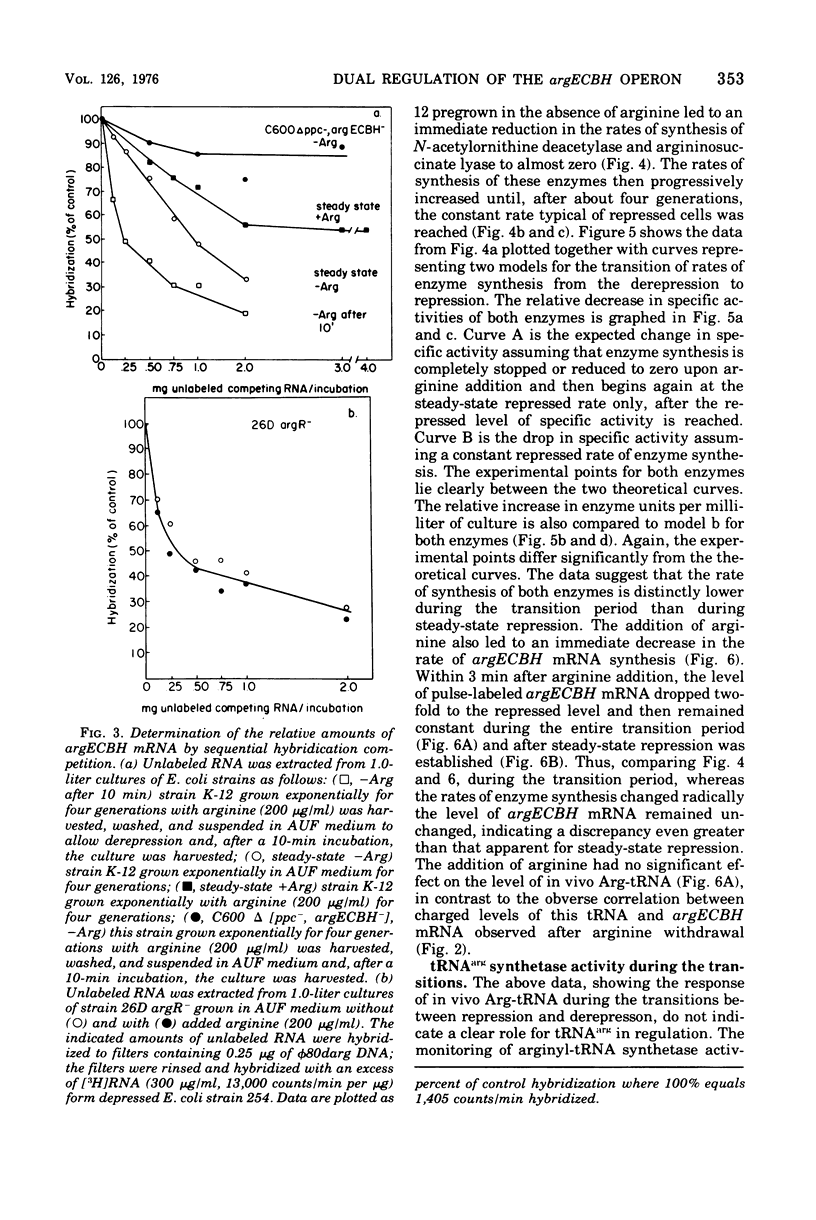
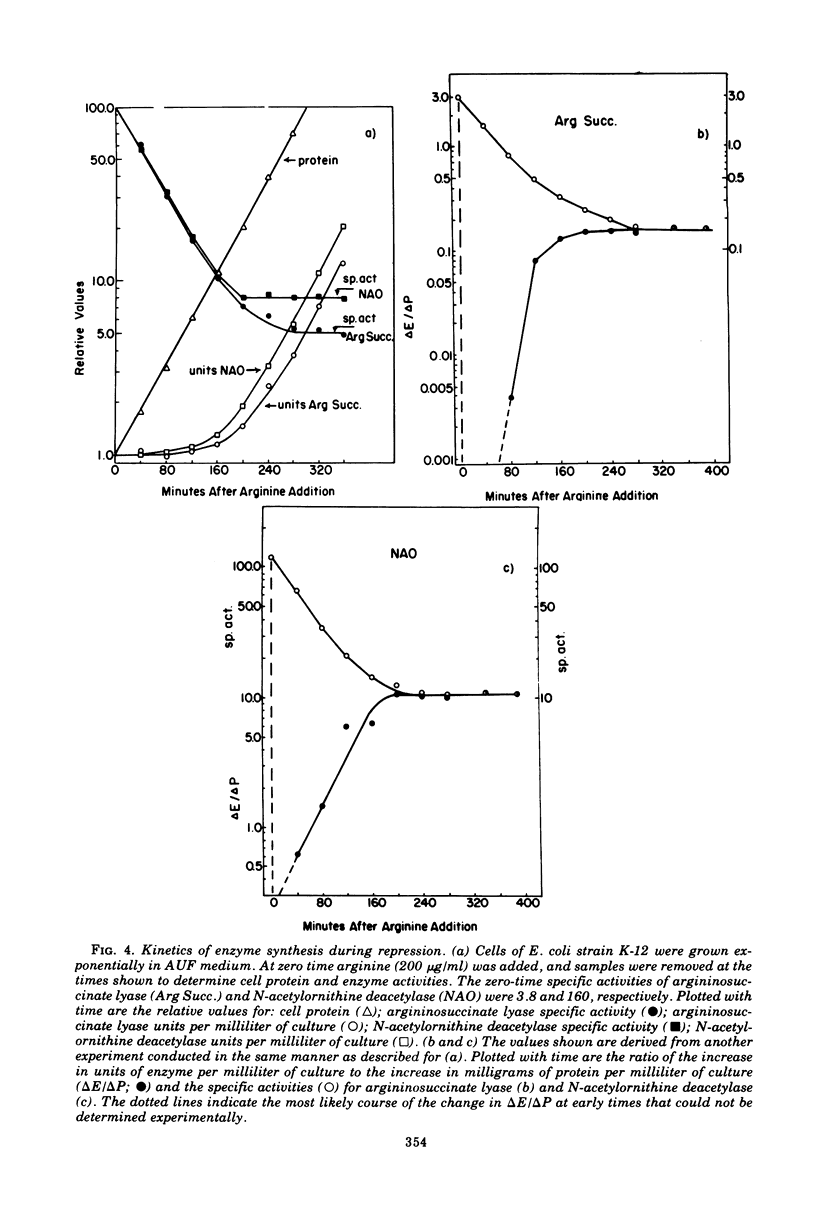
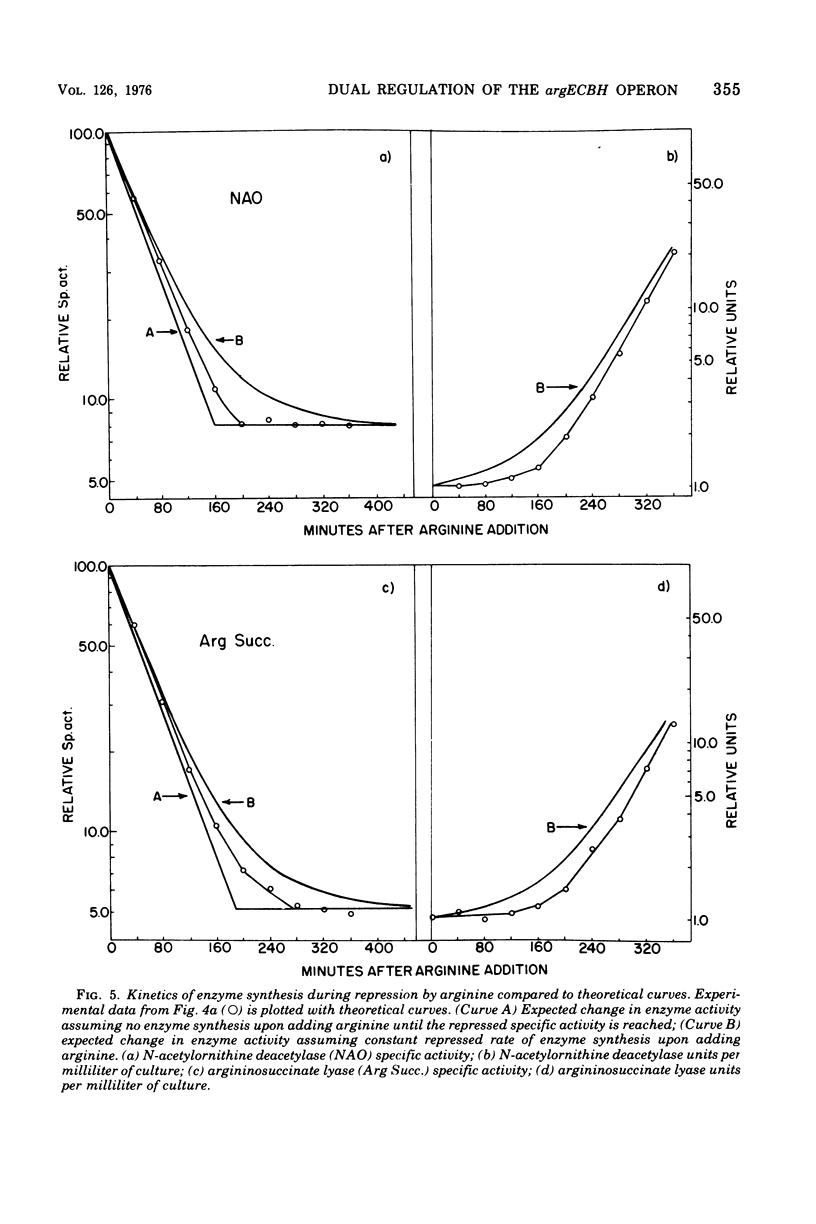
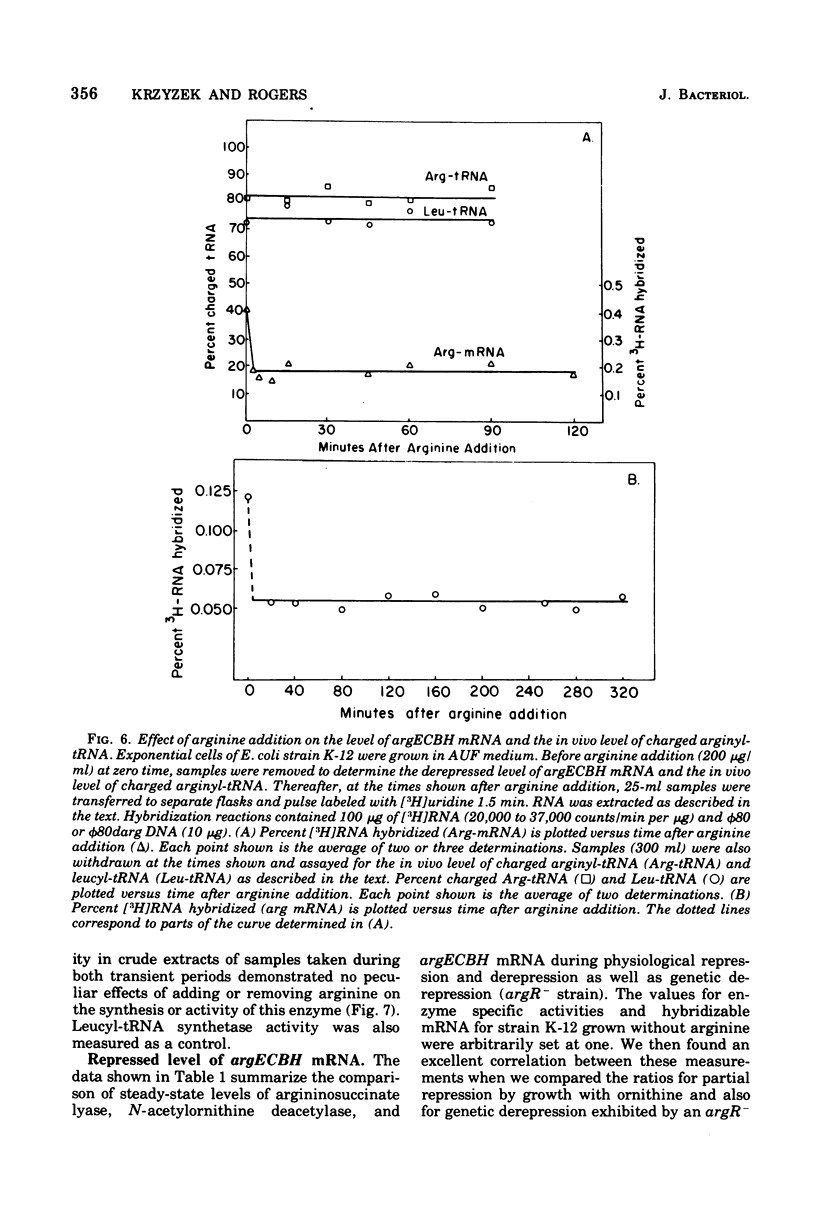
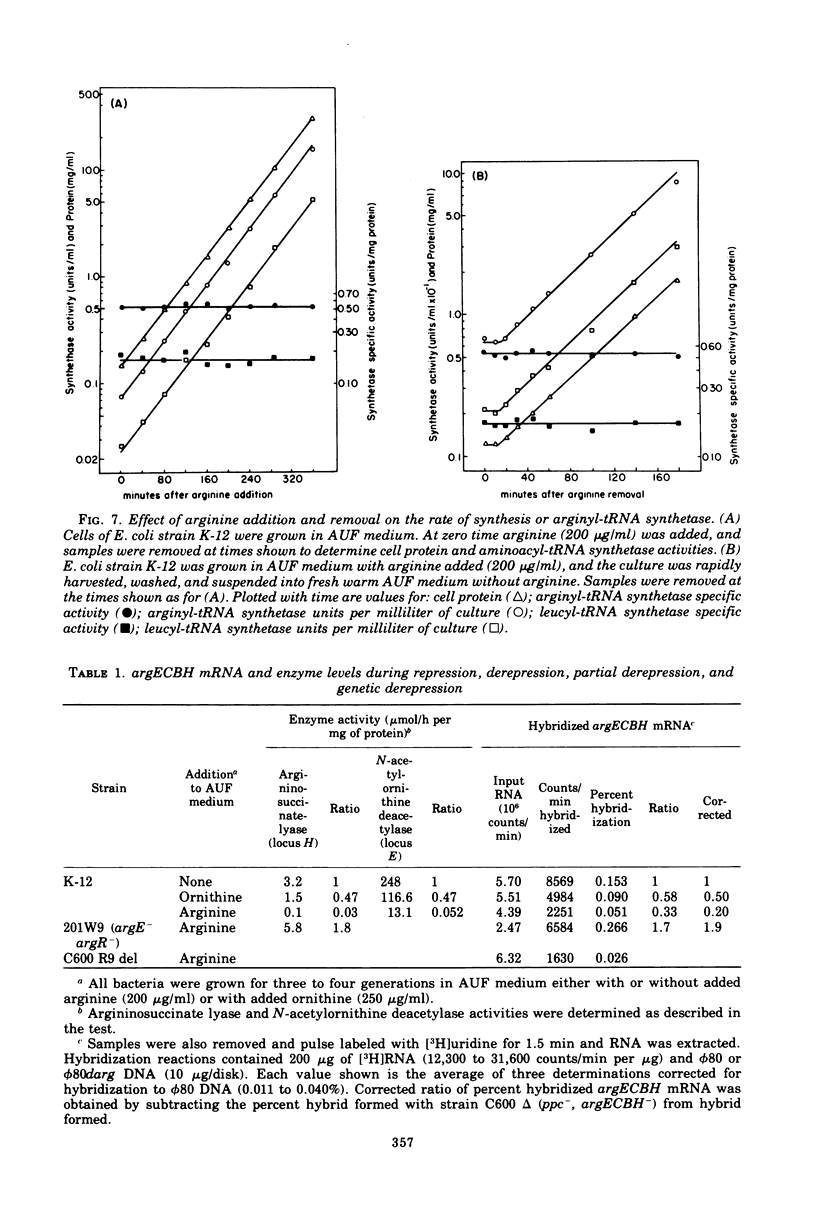
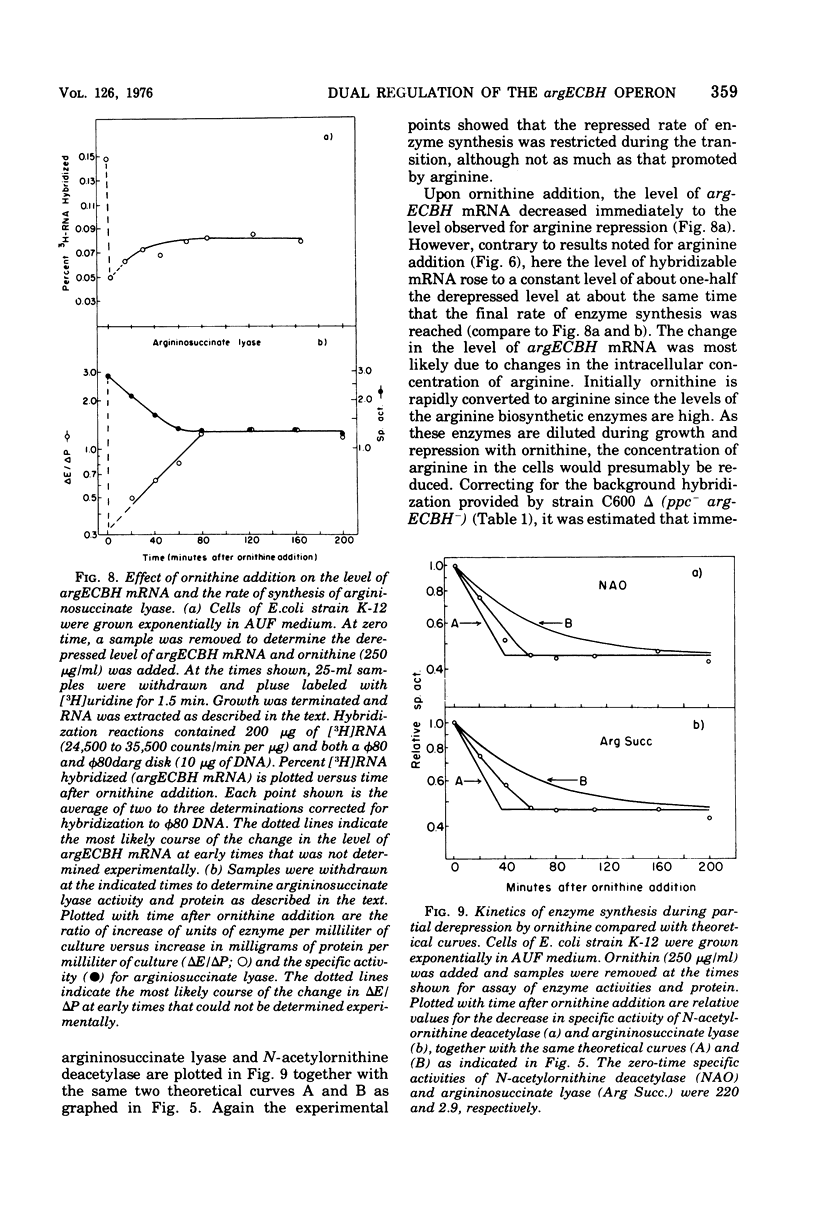
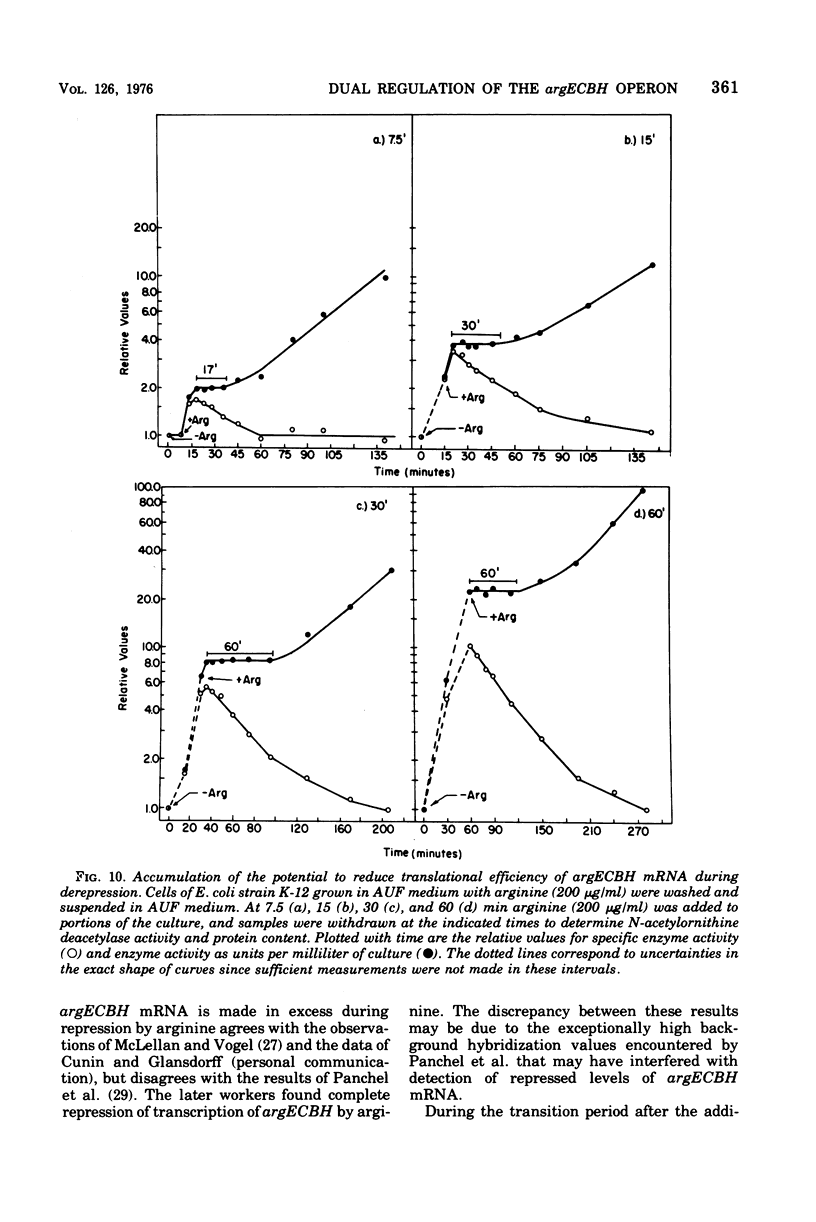
The correlation between the level of messenger ribonucleic acid (mRNA) specific for the argECBH gene cluster (argECBH mRNA) measured by ribonucleic acid-deoxyribonucleic acid (RNA-DNA) hybridization and the rates of synthesis of N-acetylornithine deacetylase (argE enzyme) and of argininosuccinate lyase (argH enzyme) of Escherichia coli strain K-12 were determined for steady-state growth with and without added L-arginine and during the transition periods between these two states. During the transient period after arginine removal (transient derepression), the synthesis of enzymes argE and argH was initially three to five times greater than the steady-state derepressed rate finally reached 50 min later. The level of argECHB mRNA correlated well both quantitatively and temporally with the rates of enzyme synthesis during this transition. The level of in vivo charged arginyl-transfer RNA (tRNAarg), monitored simultaneously, was initially only 5 to 10% and gradually increased to a final level of 80% after 45 min. During the transient period after arginine addition (transient repression), the rates of synthesis of enzymes argE and argH decreased to almost zero and gradually reached steady-state repressed rates after about 180 min. The argECBH mRNA level remained constant at the steady-state repressed level throughout transient repression, revealing a discontinuity between the level of this mRNA and rates of enzyme synthesis. A similar discrepancy was noted during the transition after ornithine addition. In vivo charged tRNAarg remained constant at 80% during this transition. After removal of arginine, the zero-level transient enzyme synthesis developed after only 7.5 min of arginine deprivation and was maximum after 30 min. The results suggest an accumulation of a molecule regulated by arginine that plays a role in transient repression. Our data indicate that arginyl-tRNA synthetase is not this molecule since its synthesis was unaffected by arginine. The ratios of steady-state argE and argH enzyme synthesis without arginine to that with arginine were 12 and 20, respectively, whereas the similar ratio for argECBH mRNA was 2 to 3. The repressed level of argECBH mRNA was not affected by attempts to repress or derepress the ppc+ gene (carried on the DNA used for hybridization), and the repressed level of argECBH mRNA was lowered about 50% in cells carrying an internal argBH deletion. These data taken together indicate the presence of an excess of untranslated argECBH mRNA during both transient and steady-state repression by arginine. Thus, a second regulatory mechanism, not yet defined, appears to play an important role in arginine regulation of enzyme synthesis.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasi F., Bruni C. B., Avitabile A., Deeley R. G., Goldberger R. F., Meyers M. M. Inhibition of transcription of the histidine operon in vitro by the first enzyme of the histidine pathway. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2692–2696. doi: 10.1073/pnas.70.9.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis T. F., Maas W. K. Studies on the mechanism of repression of arginine biosynthesis in Escherichia coli. IV. Further studies on the role of arginine transfer RNA repression of the enzymes of arginine biosynthesis. J Mol Biol. 1971 Nov 28;62(1):179–188. doi: 10.1016/0022-2836(71)90138-0. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Cunin R., Boyen A., Glansdorff N., Piérard A. Accumulation of arginine precursors in Escherichia coli: effects on growth, enzyme repression, and application to the forward selection of arginine auxotrophs. J Bacteriol. 1975 Sep;123(3):898–904. doi: 10.1128/jb.123.3.898-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Boyen A., Pouwels P., Glansdorff N., Crabeel M. Parameters of gene expression in the bipolar argECBH operon of E. coli K12. The question of translational control. Mol Gen Genet. 1975 Sep 15;140(1):51–60. doi: 10.1007/BF00268988. [DOI] [PubMed] [Google Scholar]
- Cunin Raymond, Glansdorff Nicolas. Messenger RNA from arginine and phosphoenolpyruvate carboxylase genes in arg R+ and arg R(-) strains of E. coli K-12. FEBS Lett. 1971 Oct 15;18(1):135–137. doi: 10.1016/0014-5793(71)80428-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Repression of enzymes of arginine biosynthesis by L-canavanine in arginyl-transfer ribonucleic acid synthetase mutants of Escherichia coli. J Bacteriol. 1972 Oct;112(1):102–113. doi: 10.1128/jb.112.1.102-113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Roles of arginine and canavanine in the synthesis and repression of ornithine transcarbamylase by Escherichia coli. J Bacteriol. 1968 Aug;96(2):409–420. doi: 10.1128/jb.96.2.409-420.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- Glansdorf N., Sand G. Coordination of enzyme synthesis in the arginine pathway of Escherichia coli K-12. Biochim Biophys Acta. 1965 Oct 11;108(2):308–311. doi: 10.1016/0005-2787(65)90016-x. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Horn P. C., Hopwood D. A., Maas W. K., DeDeken R. Studies on the mechanism of repression of arginine biosynthesis in Escherichia coli. 3. Repression of enzymes of arginine biosynthesis in arginyl-tRNA synthetase mutants. J Mol Biol. 1968 Jul 14;35(1):83–93. doi: 10.1016/s0022-2836(68)80038-5. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;117(4):337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. I. The genetic evidence. J Mol Biol. 1969 Jan 14;39(1):73–87. doi: 10.1016/0022-2836(69)90334-9. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Mapping the gene determining ornithine transcarbamylase and its operator in Escherichia coli B. J Bacteriol. 1971 Nov;108(2):645–651. doi: 10.1128/jb.108.2.645-651.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Rogers P. Effect of arginine on the stability and size of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1976 Apr;126(1):365–376. doi: 10.1128/jb.126.1.365-376.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R., Rogers P. Arginine control of transcription of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1972 Jun;110(3):945–954. doi: 10.1128/jb.110.3.945-954.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Leisinger T., Haas D. N-Acetylglutamate synthase of Escherichia coli regulation of synthesis and activity by arginine. J Biol Chem. 1975 Mar 10;250(5):1690–1693. [PubMed] [Google Scholar]
- Leisinger T., Vogel R. H., Vogel H. J. Repression-dependent alteration of an arginine enzyme in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Oct;64(2):686–692. doi: 10.1073/pnas.64.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K., MAAS R., WIAME J. M., GLANSDORFF N. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. I. DOMINANCE OF REPRESSIBILITY IN ZYGOTES. J Mol Biol. 1964 Mar;8:359–364. doi: 10.1016/s0022-2836(64)80199-6. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. STUDIES ON THE MECHANISM OF REPRESSION OF ARGININE BIOSYNTHESIS IN ESCHERICHIA COLI. II. DOMINANCE OF REPRESSIBILITY IN DIPLOIDS. J Mol Biol. 1964 Mar;8:365–370. doi: 10.1016/s0022-2836(64)80200-x. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational repression in the arginine system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1703–1709. doi: 10.1073/pnas.67.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal C. J., Bagchee S. N., Guha A. Divergent orientation of transcription from the arginine gene ECBH cluster of Escherichia coli. J Bacteriol. 1974 Feb;117(2):675–680. doi: 10.1128/jb.117.2.675-680.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels P. H., Cunin R., Glansdorff N. Letter: Divergent transcription in the argECBH cluster of genes in Escherichia coli K12. J Mol Biol. 1974 Mar;83(3):421–424. doi: 10.1016/0022-2836(74)90288-5. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Rogers P., Kaden T. M., Toth M. Repression of Arg mRNA synthesis by L-arginine in cell-free extracts of Escherichia coli. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1284–1291. doi: 10.1016/s0006-291x(75)80369-x. [DOI] [PubMed] [Google Scholar]
- Rogers P., Krzyzek R., Kaden T. M., Arfman E. Effect of arginine and canavanine on arginine messenger RNA synthesis. Biochem Biophys Res Commun. 1971 Sep;44(5):1220–1226. doi: 10.1016/s0006-291x(71)80216-4. [DOI] [PubMed] [Google Scholar]
- Sens D., James E. Regulation of argF mRNA synthesis, performed in vitro. Biochem Biophys Res Commun. 1975 May 5;64(1):169–174. doi: 10.1016/0006-291x(75)90234-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urm E., Yang H., Zubay G., Kelker N., Maas W. In vitro repression of n- -acetyl-L-ornithinase synthesis in Escherichia coli. Mol Gen Genet. 1973;121(1):1–7. doi: 10.1007/BF00353688. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Williams A. L., Williams L. S. Control of arginine biosynthesis in Escherichia coli: characterization of arginyl-transfer ribonucleic acid synthetase mutants. J Bacteriol. 1973 Mar;113(3):1433–1441. doi: 10.1128/jb.113.3.1433-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. S. Control of arginine biosynthesis in Escherichia coli: role of arginyl-transfer ribonucleic acid synthetase in repression. J Bacteriol. 1973 Mar;113(3):1419–1432. doi: 10.1128/jb.113.3.1419-1432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



