Abstract
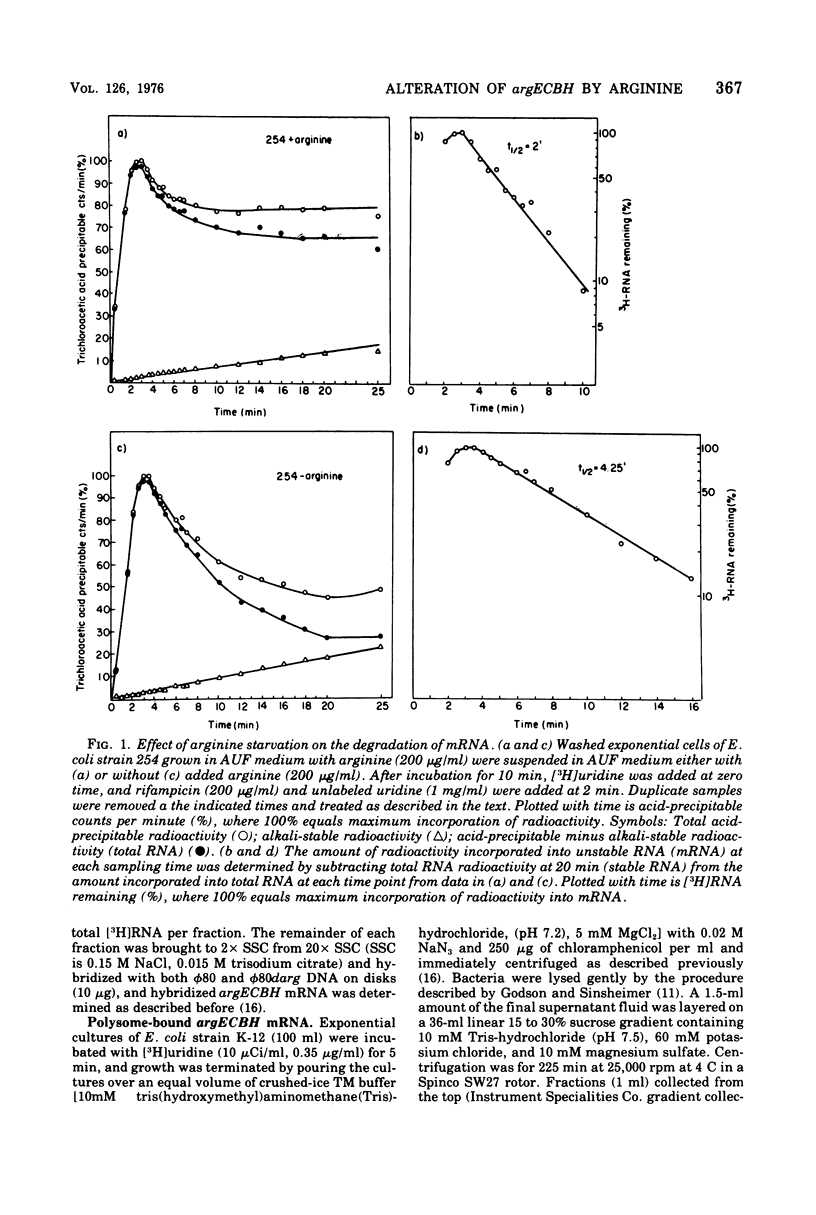
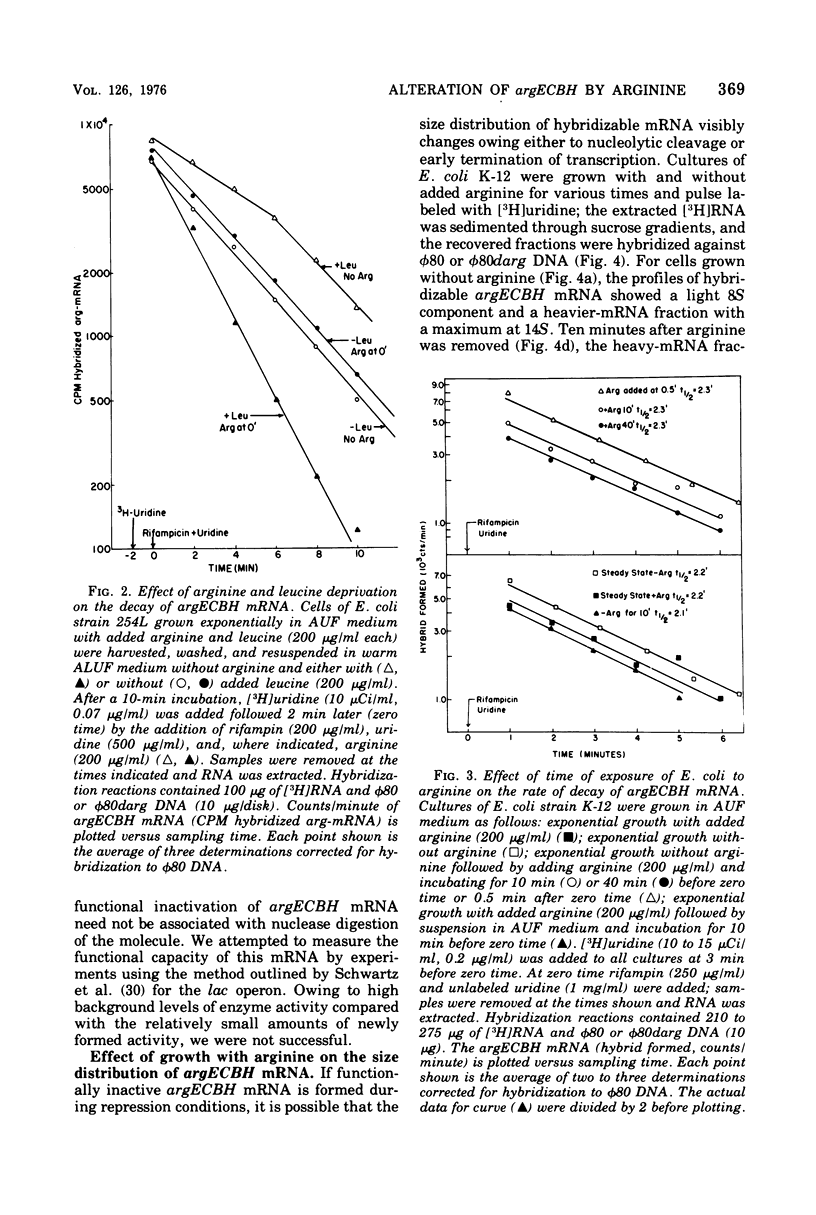
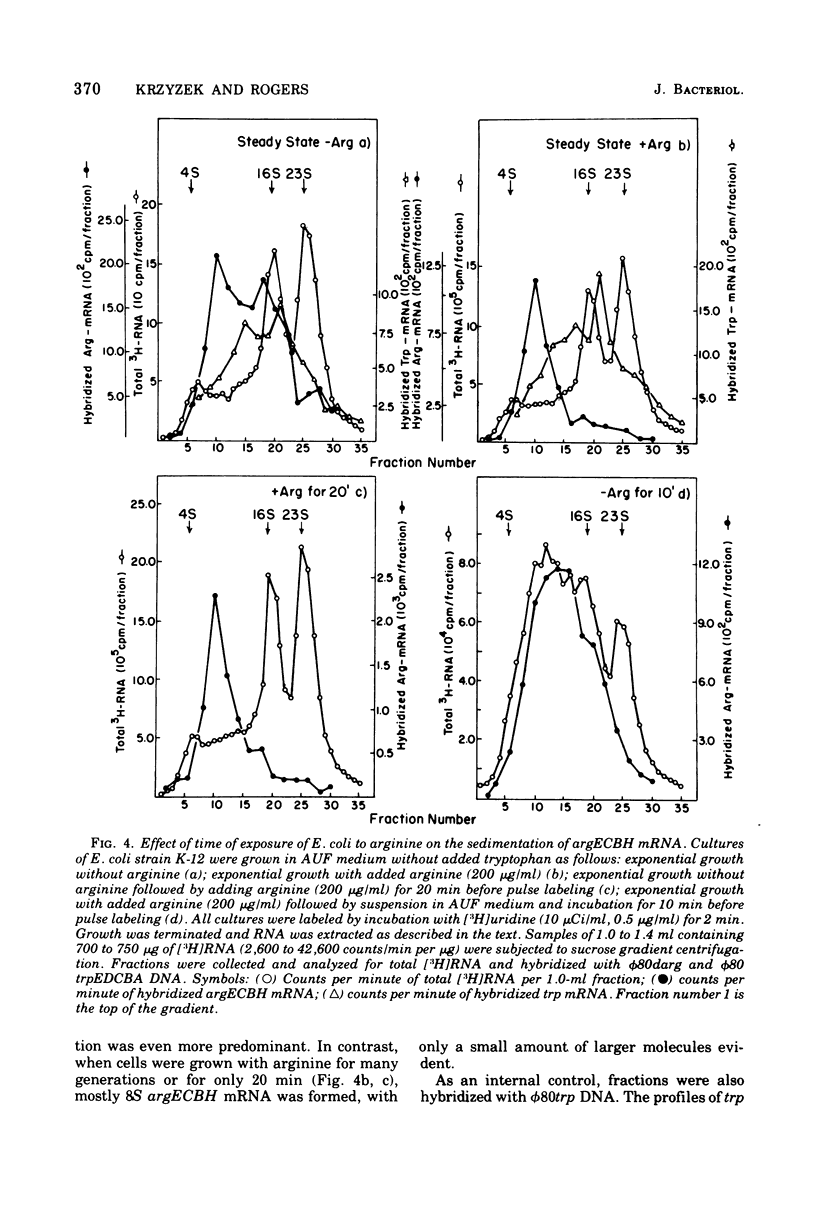
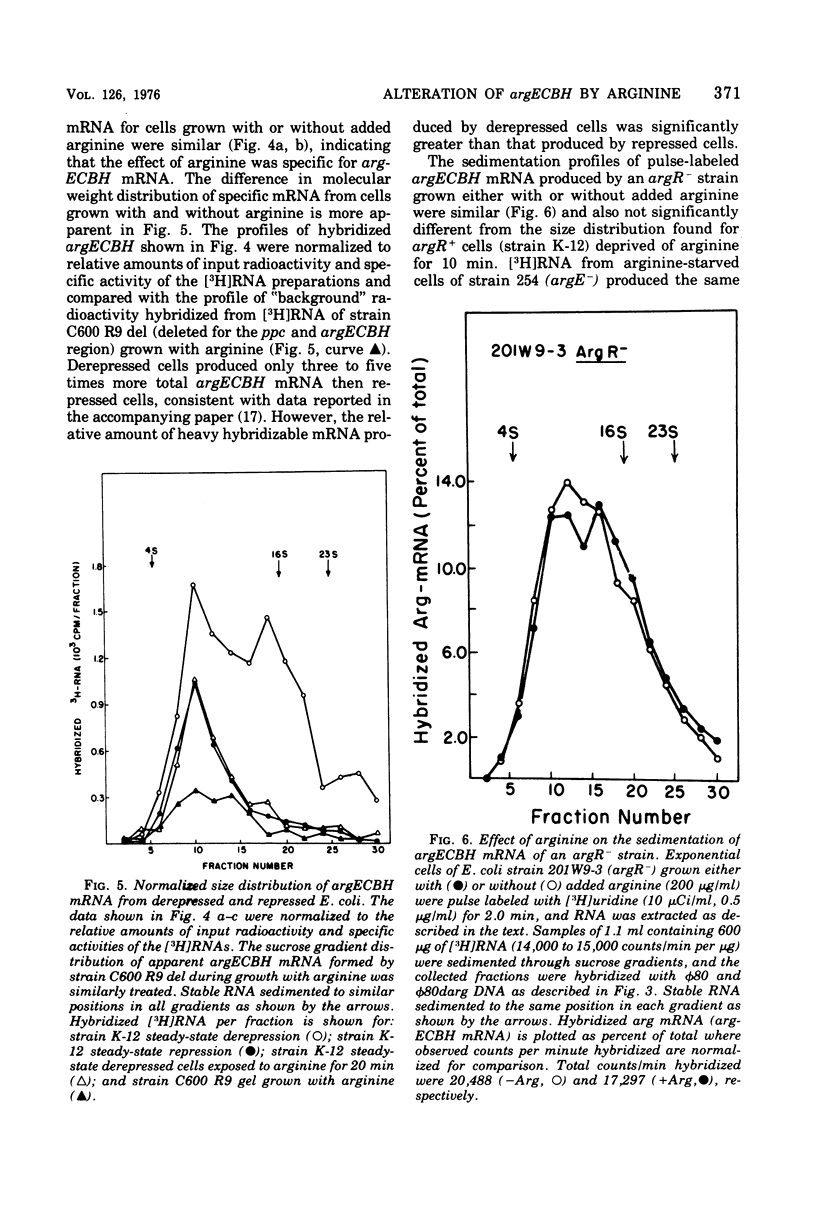
The chemical stability of argECBH messenger ribonucleic acid (mRNA) produced by Escherichia coli was found to be unaltered during steady-state repression by arginine. During extreme arginine deprivation, the increase in argECBH mRNA stability was related to general effects of amino acid starvation on mRNA stability. Thus a mechanism whereby argECBH gene expression is regulated by altering the decay rate of this mRNA is not consistent with our data. Sucrose gradient analysis followed by hybridization revealed that both heavy (14S) and light (8S) components of argECBH mRNA were produced by cells of E. coli K-12 grown without arginine, whereas predominantly light (8S) mRNA was produced by cells grown with arginine. A functional argR gene and the EC portion of the argECBH cluster were found essential for the arginine restriction of heavy-mRNA production. Experiments suggest that light argECBH mRNA did not arise from heavy message, and 8u% of both light and heavy mRNA was found bound to ribosomes. The data appear most consistent with the notion that a second site of control by arginine regulates the amounts of light and heavy arginine mRNA in the cell either by early termination of transcription or by endonucleolytic processing. Consideration of these data in conjunction with those of the accompanying report (Krzyzek and Rogers, 1976) permits the tentative conclusion that light argECBH mRNA is not translated into active enzymes and is thus responsible for the discrepancy between the high content of hybridizable mRNA and low rates of enzyme synthesis found during arginine repression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi A., Spahr P. F. Nucleotide sequence at the binding site for coat protein on RNA of bacteriophage R17. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3033–3037. doi: 10.1073/pnas.69.10.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Blundell M., Kennell D. Evidence for endonucleolytic attack in decay of lac messenger RNA in Escherichia coli. J Mol Biol. 1974 Feb 25;83(2):143–161. doi: 10.1016/0022-2836(74)90385-4. [DOI] [PubMed] [Google Scholar]
- Boy E., Theze J., Patte J. C. Transient regulation of enzyme synthesis in Escherichia coli. Mol Gen Genet. 1973;121(1):77–81. doi: 10.1007/BF00353695. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Yaniv M., Yanofsky C. Nucleotide sequences from messenger RNA transcribed from the operator-proximal portion of the tryptophan operon of Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):163–177. doi: 10.1016/0022-2836(73)90105-8. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Repression of enzymes of arginine biosynthesis by L-canavanine in arginyl-transfer ribonucleic acid synthetase mutants of Escherichia coli. J Bacteriol. 1972 Oct;112(1):102–113. doi: 10.1128/jb.112.1.102-113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faanes R., Rogers P. Roles of arginine and canavanine in the synthesis and repression of ornithine transcarbamylase by Escherichia coli. J Bacteriol. 1968 Aug;96(2):409–420. doi: 10.1128/jb.96.2.409-420.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Use of Brij lysis as a general method to prepare polyribosomes from Escherichia coli. Biochim Biophys Acta. 1967 Dec 19;149(2):489–495. doi: 10.1016/0005-2787(67)90176-1. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;117(4):337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- Kasai T. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature. 1974 Jun 7;249(457):523–527. doi: 10.1038/249523a0. [DOI] [PubMed] [Google Scholar]
- Kennell D., Bicknell I. Decay of messenger ribonucleic acid from the lactose operon of Escherichia coli as a function of growth temperature. J Mol Biol. 1973 Feb 15;74(1):21–31. doi: 10.1016/0022-2836(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Weissmann C. Possible mechanism for transition of viral RNA from polysome to replication complex. Nat New Biol. 1971 May 12;231(19):42–46. doi: 10.1038/newbio231042a0. [DOI] [PubMed] [Google Scholar]
- Kryzek R. A., Rogers P. Dual regulation by arginine of the expression of the Escherichia coli argECBH operon. J Bacteriol. 1976 Apr;126(1):348–364. doi: 10.1128/jb.126.1.348-364.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R., Rogers P. Arginine control of transcription of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1972 Jun;110(3):945–954. doi: 10.1128/jb.110.3.945-954.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Tryptophan messenger translation in Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):435–447. doi: 10.1016/0022-2836(70)90153-1. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational and transcriptional components of repression by arginine in Escherichia coli. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1027–1033. doi: 10.1016/0006-291x(72)90811-x. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Vogel H. J. Translational repression in the arginine system of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1703–1709. doi: 10.1073/pnas.67.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. Synthesis of a large precursor to ribosomal RNA in a mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3361–3365. doi: 10.1073/pnas.70.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace B., Peterson R. L., Pace N. R. Formation of all stable RNA species in Escherichia coli by posttranscriptional modification. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1097–1104. doi: 10.1073/pnas.65.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal C. J., Bagchee S. N., Guha A. Divergent orientation of transcription from the arginine gene ECBH cluster of Escherichia coli. J Bacteriol. 1974 Feb;117(2):675–680. doi: 10.1128/jb.117.2.675-680.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato M. L., Bennett P. M., von Meyenburg K. Messenger ribonucleic acid synthesis and degradation in Escherichia coli during inhibition of translation. J Bacteriol. 1973 Nov;116(2):710–718. doi: 10.1128/jb.116.2.710-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels P. H., Cunin R., Glansdorff N. Letter: Divergent transcription in the argECBH cluster of genes in Escherichia coli K12. J Mol Biol. 1974 Mar;83(3):421–424. doi: 10.1016/0022-2836(74)90288-5. [DOI] [PubMed] [Google Scholar]
- Ray P. N., Pearson M. L. Evidence for post-transcriptional control of the morphogenetic genes of bacteriophage lambda. J Mol Biol. 1974 May 5;85(1):163–175. doi: 10.1016/0022-2836(74)90135-1. [DOI] [PubMed] [Google Scholar]
- Rogers P., Kaden T. M., Toth M. Repression of Arg mRNA synthesis by L-arginine in cell-free extracts of Escherichia coli. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1284–1291. doi: 10.1016/s0006-291x(75)80369-x. [DOI] [PubMed] [Google Scholar]
- Rogers P., Krzyzek R., Kaden T. M., Arfman E. Effect of arginine and canavanine on arginine messenger RNA synthesis. Biochem Biophys Res Commun. 1971 Sep;44(5):1220–1226. doi: 10.1016/s0006-291x(71)80216-4. [DOI] [PubMed] [Google Scholar]
- Schwartz T., Craig E., Kennell D. Inactivation and degradation of messenger ribnucleic acid from the lactose operon of Escherichia coli. J Mol Biol. 1970 Dec 14;54(2):299–311. doi: 10.1016/0022-2836(70)90431-6. [DOI] [PubMed] [Google Scholar]
- Silengo L. Messenger ribonucleic acid stability in relaxed and stringent Escherichia coli starved for methionine. J Bacteriol. 1973 Jul;115(1):447–449. doi: 10.1128/jb.115.1.447-449.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



