Abstract
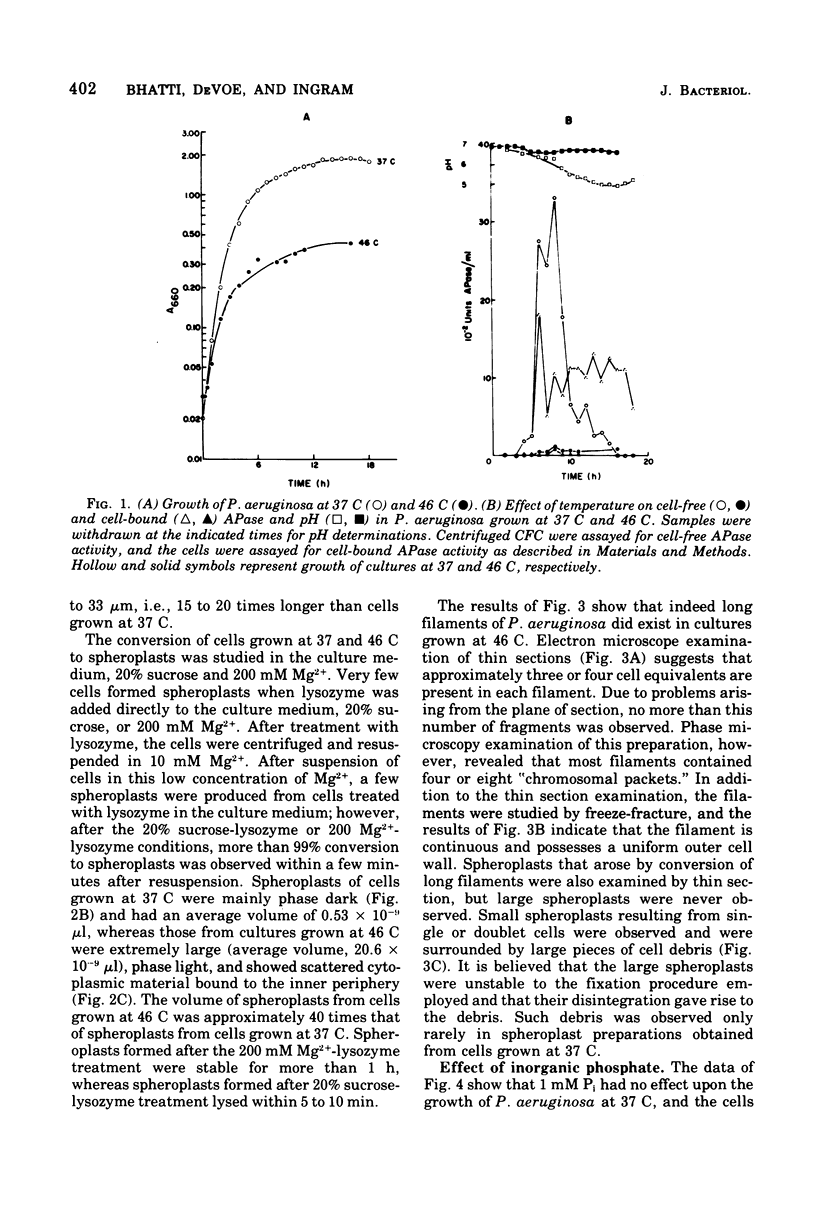
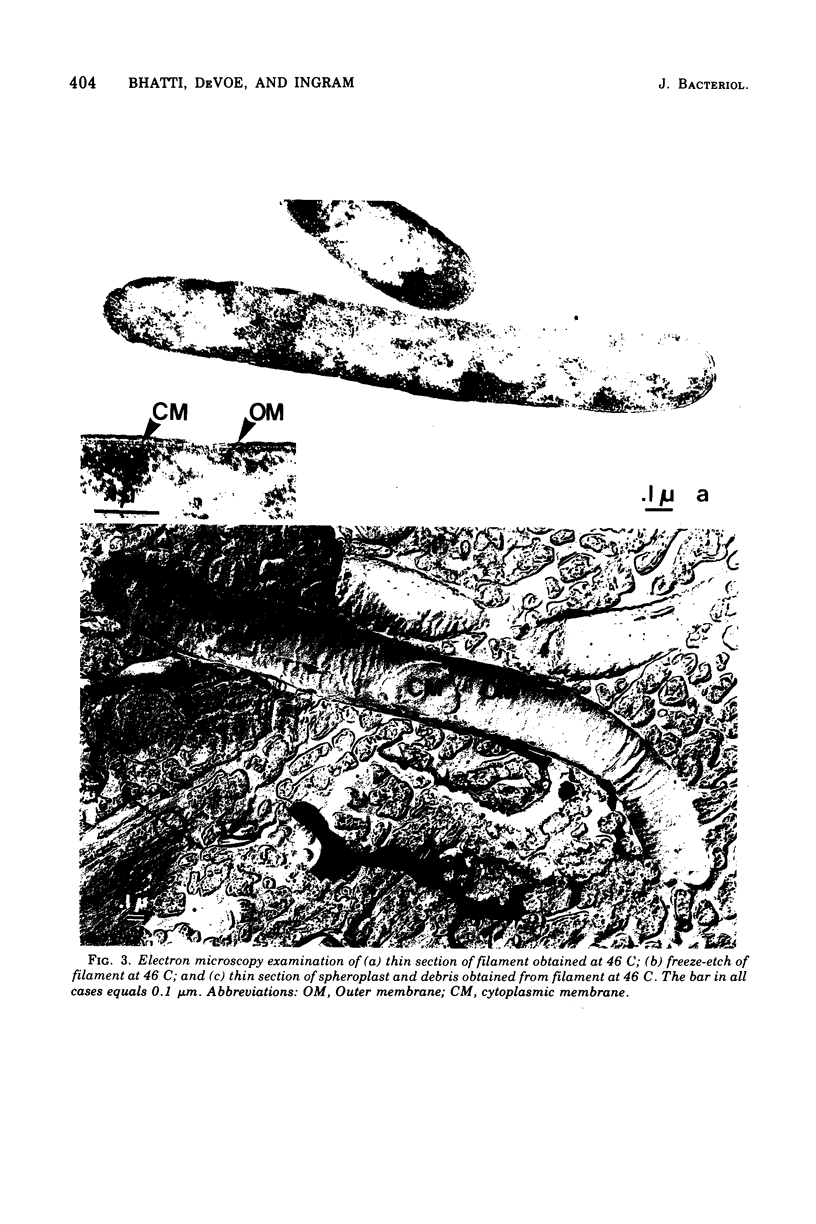
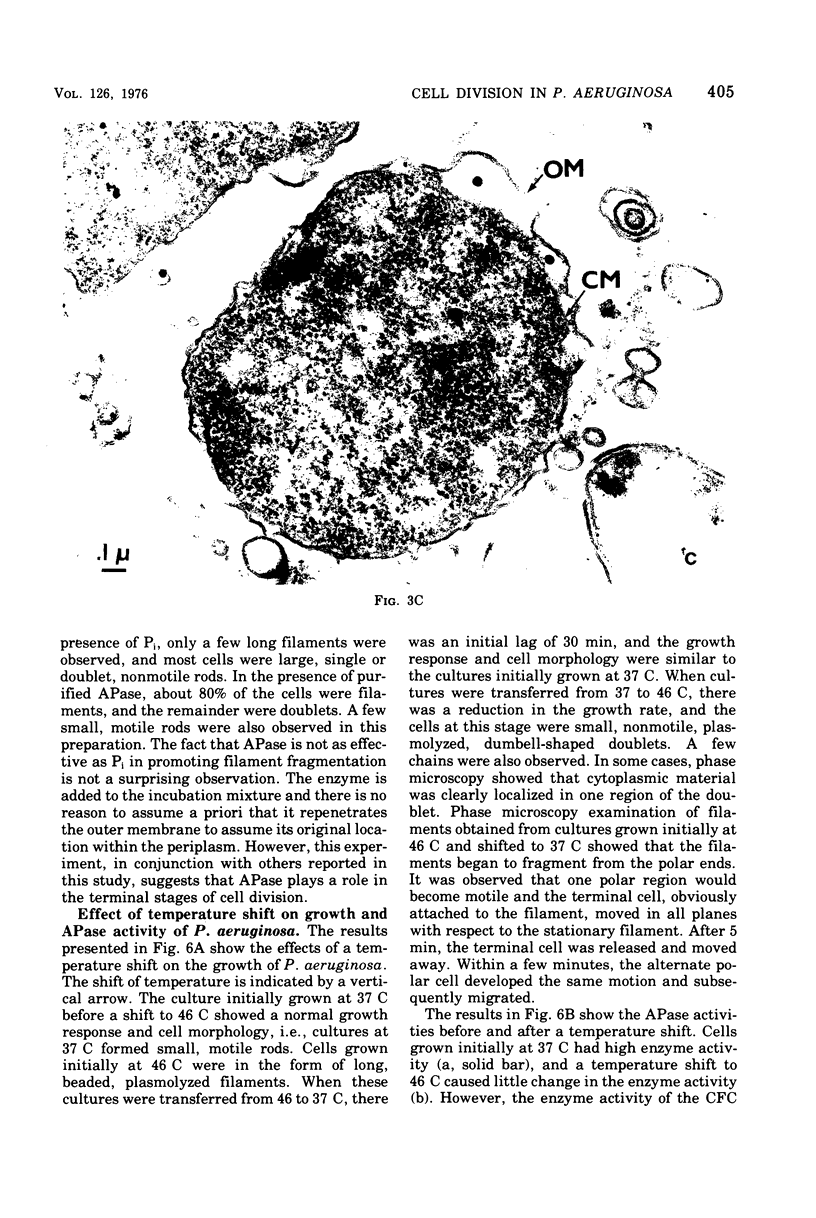
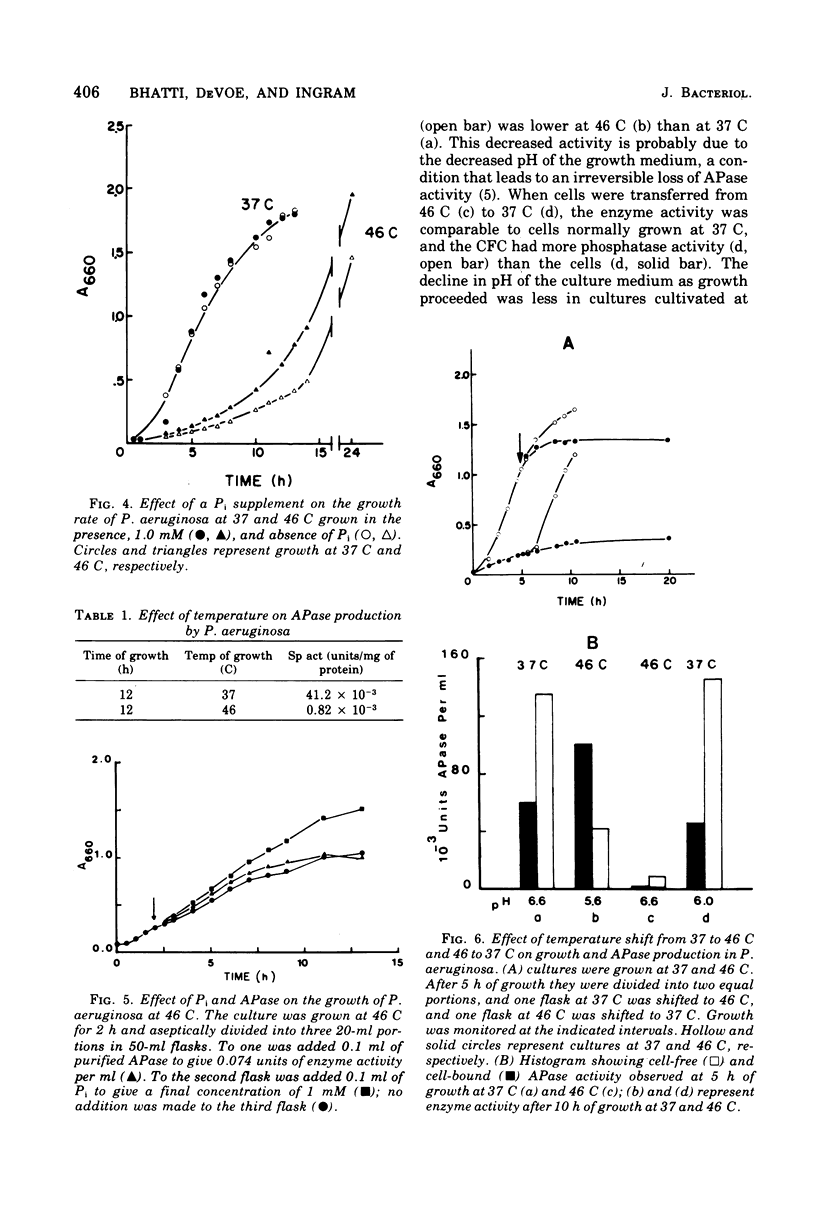
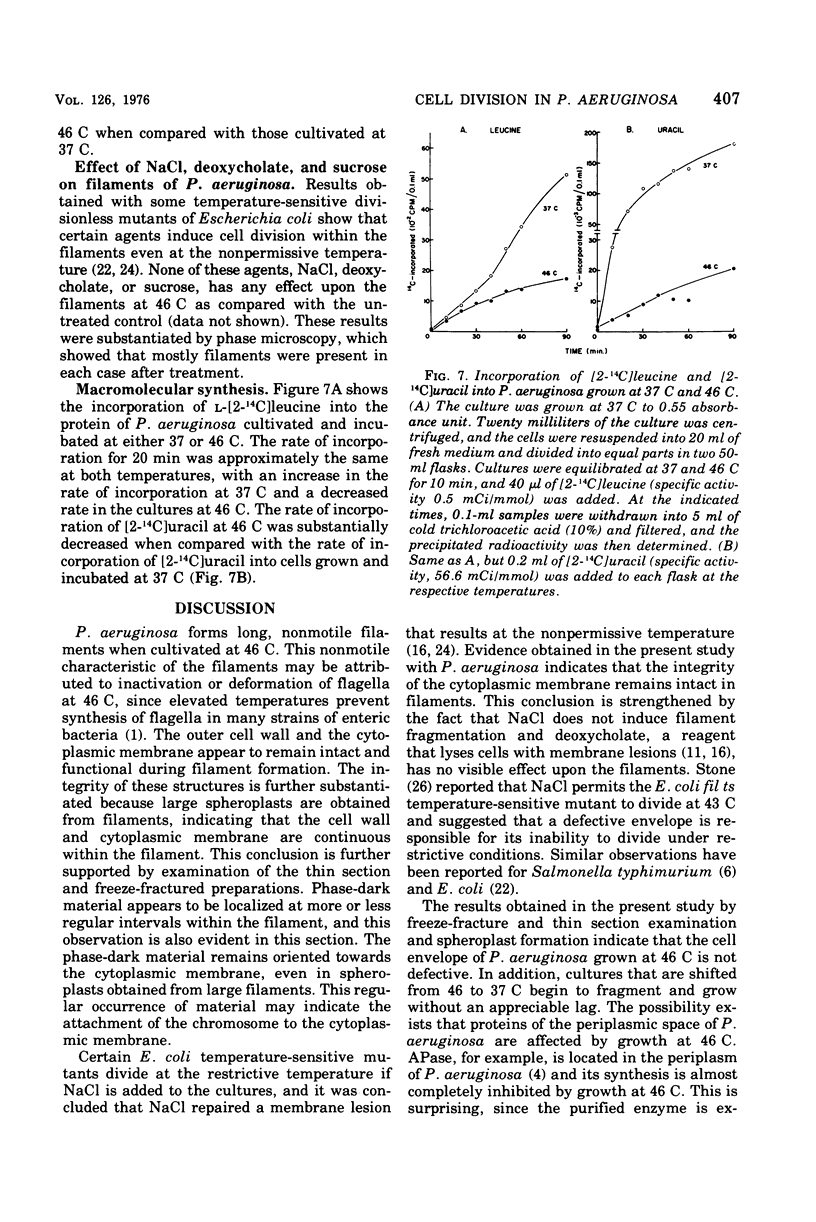
Pseudomonas aeruginosa grows at an apparent reduced rate at 46 C as compared with the rate at 37 C, when growth is measured as an increase in absorbance. Cells at 46 C are long, plasmolyzed, nonmotile filaments. The filaments contain phase-dark material that may be chromosomal in nature. When the 46 C culture is shifted to 37 C, the filaments fragment at polar ends after flagella form, and the final number of cells is equal to the number of chromosomal "packets" observed within the filament. The outer envelope of the filament appears to be structurally complete as determined by biochemical, thin section, and freeze-etch examination. When filaments are treated with lysozyme, they form large spheroplasts, suggesting that the outer wall and the cytoplasmic membrane are continuous within the filament. Filaments produce little or no periplasm-located alkaline phosphatase (APase), but activity appears immediately after a shift to 37 C. Cells grown at 37 C and shifted to 46 C remain as single, nonmotile, rods or doublets, and the APase formed at 37 C remains stable at 46 C. The addition of APase or inorganic phosphate is partially or completely effective as an inducer of filament fragmentation at 46 C. The results suggest that periplasm-located APase is an important enzyme in the final stages of cell division when P. aeruginosa is cultured on inorganic phosphate-limiting media.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967 Feb;46(2):175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- Ahmed N., Rowbury R. J. Temperature-sensitive cell division component in a mutant of Salmonella typhimurium. J Gen Microbiol. 1971 Jul;67(1):107–115. doi: 10.1099/00221287-67-1-107. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Day D. F., Costerton J. W., Ingram J. M. Alkaline phosphatase subunits in the culture filtrate of Pseudomonas aeruginosa. Can J Biochem. 1972 Mar;50(3):268–276. doi: 10.1139/o72-038. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Alkaline phosphatase localization and spheroplast formation of Pseudomonas aeruginosa. Can J Microbiol. 1970 Dec;16(12):1319–1324. doi: 10.1139/m70-218. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieśla Z., Bagdassarian M. Imparired DNA synthesis and envelope defect in a mutant of Salmonella typhimurium. Mol Gen Genet. 1972;116(2):126–138. doi: 10.1007/BF00582222. [DOI] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. F., Ingram J. M. In vitro studies of an alkaline phosphatase-cell wall complex from Pseudomonas aeruginosa. Can J Microbiol. 1975 Jan;21(1):9–16. doi: 10.1139/m75-002. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W., Costerton J. W., MacLeod R. A. Demonstration by freeze-etching of a single cleavage plane in the cell wall of a gram-negative bacterium. J Bacteriol. 1971 May;106(2):659–671. doi: 10.1128/jb.106.2.659-671.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E., Storm D. W. Ultrastructural studies on the fate of group B meningococci in human peripheral blood leukocytes. Can J Microbiol. 1973 Nov;19(11):1355–1359. doi: 10.1139/m73-218. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M. Micrococcus lysodeikticus bacterial walls as a substrate specific for the autolytic glycosidase of Bacillus subtilis. J Bacteriol. 1973 May;114(2):804–813. doi: 10.1128/jb.114.2.804-813.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni G. D., Inniss W. E. Thermally caused filament formation in the psychrophile Bacillus insolitus. Can J Microbiol. 1973 May;19(5):581–584. doi: 10.1139/m73-095. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hitchins A. D., Sadoff H. L. Properties of a thermosensitive asporogenous filamentous mutant of Bacillus megaterium. J Bacteriol. 1974 Jun;118(3):1167–1175. doi: 10.1128/jb.118.3.1167-1175.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Strominger J. L. Penicillin-resistant temperature-sensitive mutants of Escherichia coli which synthesize hypo- or hyper-cross-linked peptidoglycan. J Bacteriol. 1974 Feb;117(2):568–577. doi: 10.1128/jb.117.2.568-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagai K., Kaneko H., Tamura G. Thermosensitive mutant of Escherichia coli requiring new protein synthesis to recover cellular division ability. Biochem Biophys Res Commun. 1971 Feb 19;42(4):669–675. doi: 10.1016/0006-291x(71)90540-7. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Otsuji N., Iyehara H., Hideshima Y. Isolation and characterization of an Escherichia coli ruv mutant which forms nonseptate filaments after low doses of ultraviolet light irradiation. J Bacteriol. 1974 Feb;117(2):337–344. doi: 10.1128/jb.117.2.337-344.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E. Z., Davis B. D. Growth rate of Escherichia coli at elevated temperatures: limitation by methionine. J Bacteriol. 1971 Aug;107(2):391–396. doi: 10.1128/jb.107.2.391-396.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Pardee A. B. Accumulation of a protein required for division during the cell cycle of Escherichia coli. J Bacteriol. 1970 Mar;101(3):901–909. doi: 10.1128/jb.101.3.901-909.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. B. Regulation of cell division in a temperature-sensitive division mutant of Escherichia coli. J Bacteriol. 1973 Nov;116(2):741–750. doi: 10.1128/jb.116.2.741-750.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Blumberg P. M., Suginaka H., Umbreit J., Wickus G. G. How penicillin kills bacteria: progress and problems. Proc R Soc Lond B Biol Sci. 1971 Dec 31;179(1057):369–383. doi: 10.1098/rspb.1971.0103. [DOI] [PubMed] [Google Scholar]