Abstract
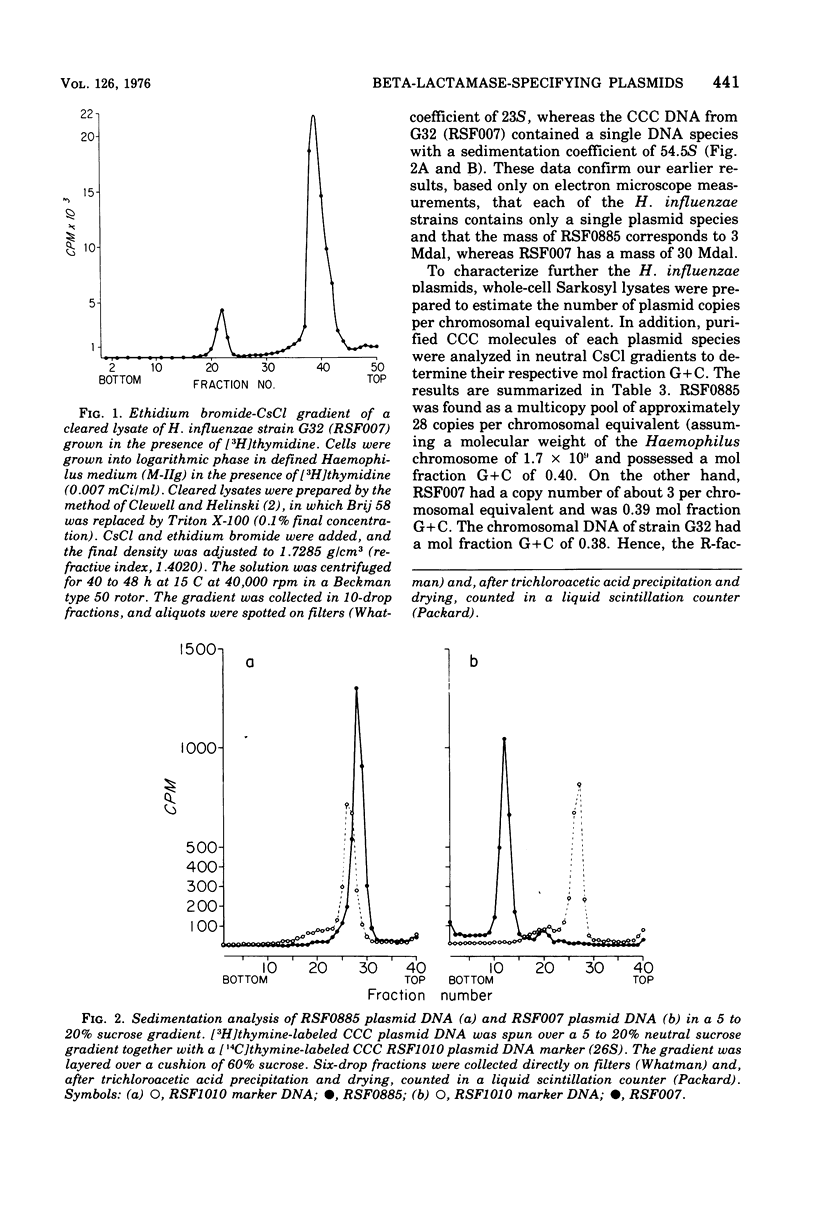
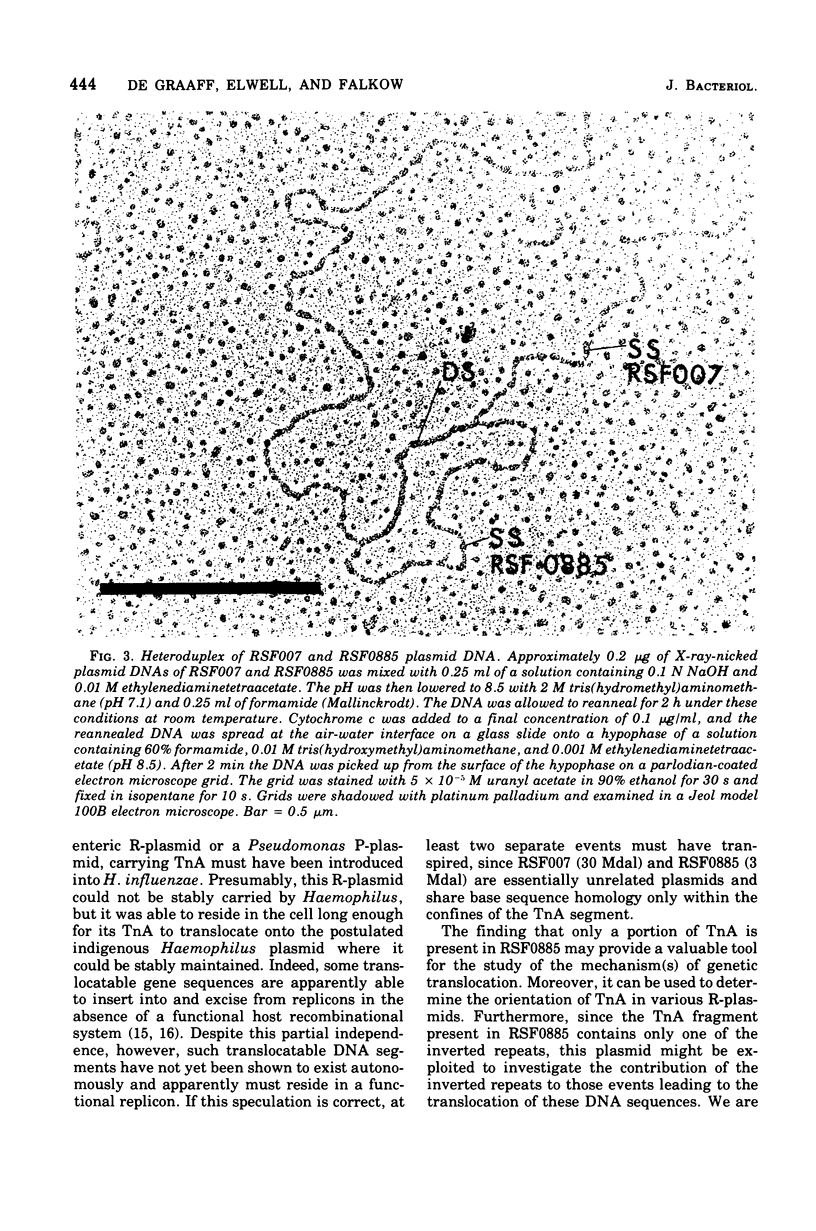
The molecular nature of two beta-lactamase-specifying plasmids isolated from two separate ampicillin-resistant Haemophilus influenzae type b strains was examined. A 30 X 10(6)-dalton (30-Mdal) plasmid (RSF007) had a copy number of approximately 3 per chromosomal equivalent and a mole fraction guanine plus cytosine content of 0.39. By heteroduplex analysis the 30-Mdal plasmid was found to contain the entire ampicillin translocation DNA segment (TnA) found on R factors of enteric origin. A 3.0-Mdal plasmid (RSF0885) was found as a multicopy pool of approximately 28 copies per chromosomal equivalent, had a mole fraction guanine plus cytosine content of 0.40, and contained only about one-third of the transposable TnA sequence. RSF007 and RSF0885 appeared to be unrelated plasmids in that they share base sequence homology only within the confines of the TnA segment. The 3.0-Mdal Haemophilus plasmid was used to transform E. coli to ampicillin resistance but was found to be unstable in this host in the absence of antibiotic. The possibility that R-plasmids arose in Haemophilus by the translocation of TnA from a donor R-factor onto an indigenous H. influenzae plasmid is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- So M., Crosa J. H., Falkow S. Polynucleotide sequence relationships among Ent plasmids and the relationship between Ent and other plasmids. J Bacteriol. 1975 Jan;121(1):234–238. doi: 10.1128/jb.121.1.234-238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. T., Herriott R. M. Development of competence of Haemophilus influenzae. J Bacteriol. 1965 Oct;90(4):911–920. doi: 10.1128/jb.90.4.911-920.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Survey of numerical techniques for grouping. Bacteriol Rev. 1972 Jun;36(2):261–261. doi: 10.1128/br.36.2.261-261.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van A. D., Bieth G., Bouanchaud D. H. Résistance plasmidique à la tétracycline chez Haemophilus influenzae. C R Acad Sci Hebd Seances Acad Sci D. 1975 Mar 10;280(10):1321–1323. [PubMed] [Google Scholar]
- Van A. D., Goldstein F., Acar J. F., Bouanchaud D. H. A transferable kanamycin resistance plasmid isolated from Haemophilus influenzae. Ann Microbiol (Paris) 1975 Apr;126(3):397–399. [PubMed] [Google Scholar]
- Young E. T., 2nd, Sinsheimer R. L. Vegetative bacteriophage lambda-DNA. II. Physical characterization and replication. J Mol Biol. 1967 Nov 28;30(1):165–200. doi: 10.1016/0022-2836(67)90251-3. [DOI] [PubMed] [Google Scholar]