Abstract
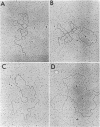
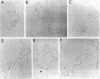
Replicating deoxyribonucleic acid (DNA) molecules of plasmid RSF1040, a deletion mutant of the conjugative R plasmid R6K, appear in the electron microscope as partially supercoiled structures with two open circular branches of equal size, although open structures with three branches, two branching points and no supercoiled regions (theta structures) were also found at a lower frequency. The partially supercoiled molecules sediment more rapidly than native covalently closed circular DNA in neutral sucrose gradients and band at a position intermediate between covalently closed circular and open circular DNA in CsClethidium bromide gradients. Electron microscope measurements of the linear EcoRI-treated replicative intermediates indicate that replication can be initiated at two sites (origins) on the plasmid DNA molecule located at about 23% (alpha) and 39% (beta) of the total genome length from an EcoRI end designated arbitrarily as the "left-hand" end of the molecule. The overall replication of RSF1040 is asymmetrically bidirectional. Replication from the alpha origin proceeds first to the "right" to a unique termination site located some 55% of the total genome length from the left-hand end of the molecule. At this point replication proceeds from the alpha origin to the "left" (i.e., opposite to the original direction of replication) until replication of the molecule is completed. Replication also proceeds from the beta origin asymmetrically to the unique terminus site.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaux P., Bourgaux-Ramoisy D. Unwinding of replicating polyoma virus DNA. J Mol Biol. 1972 Oct 14;70(3):399–413. doi: 10.1016/0022-2836(72)90548-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Nature of R-factor replication in the presence of chloramphenicol. Proc Natl Acad Sci U S A. 1975 Feb;72(2):654–658. doi: 10.1073/pnas.72.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Heffron F., Falkow S. Two replication initiation sites on R-plasmid DNA. Mol Gen Genet. 1975 Sep 15;140(1):39–50. doi: 10.1007/BF00268987. [DOI] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Replication of colicin E1 plasmid DNA in minicells from a unique replication initiation site. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2256–2259. doi: 10.1073/pnas.71.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Punch J. D. The problems of drug-resistant pathogenic bacteria. Regulation of R-factor replication in Proteus mirabilis. Ann N Y Acad Sci. 1971 Jun 11;182:201–216. doi: 10.1111/j.1749-6632.1971.tb30657.x. [DOI] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Lovett M. A., Helinski D. R. Strand and site specificity of the relaxation event for the relaxation complex of the antibiotic resistance plasmid R6K. Biochemistry. 1974 Dec 31;13(27):5484–5490. doi: 10.1021/bi00724a005. [DOI] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Sparks R. B., Helinski D. R. Bidirectional replication of plasmid R6K DNA in Escherichia coli; correspondence between origin of replication and position of single-strand break in relaxed complex. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2905–2909. doi: 10.1073/pnas.72.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Rownd R. H. Accumulation of replicating bacterial plasmid DNA during thymine limitation or hydroxyurea treatment. Mol Gen Genet. 1975 Jul 10;138(4):281–291. doi: 10.1007/BF00264797. [DOI] [PubMed] [Google Scholar]
- Roman A., Champoux J. J., Dulbecco R. Characterization of the replicative intermediates of polyoma virus. Virology. 1974 Jan;57(1):147–160. doi: 10.1016/0042-6822(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L. Replication of colicinogenic factor E 1 DNA: evidence for a discontinuous replication mechanism. Nucleic Acids Res. 1974 Sep;1(9):1153–1164. doi: 10.1093/nar/1.9.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Utilization of two distinct modes of replication by a hybrid plasmid constructed in vitro from separate replicons. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4556–4560. doi: 10.1073/pnas.71.11.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Sakakibara Y., Kakefuda T. Replication of colicin E1 plasmid DNA in cell extracts. Origin and direction of replication. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2260–2264. doi: 10.1073/pnas.71.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]