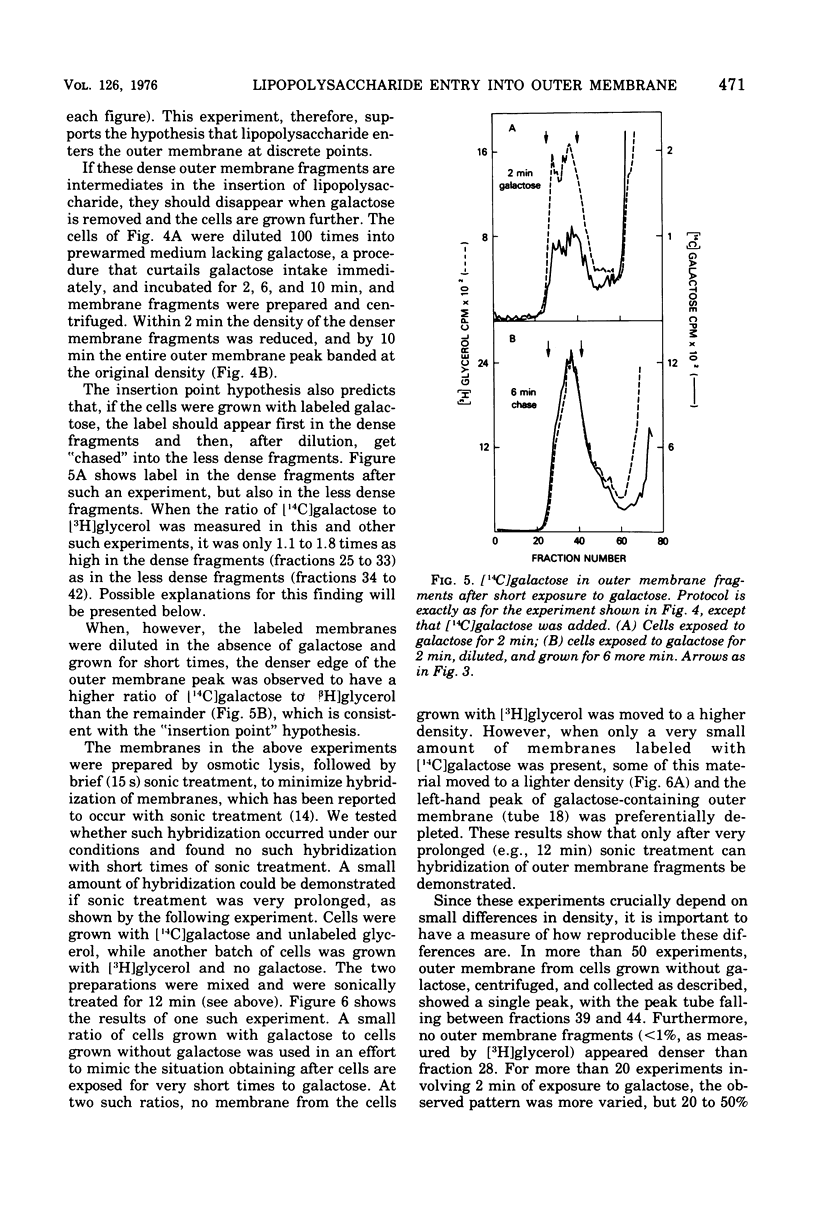
Abstract
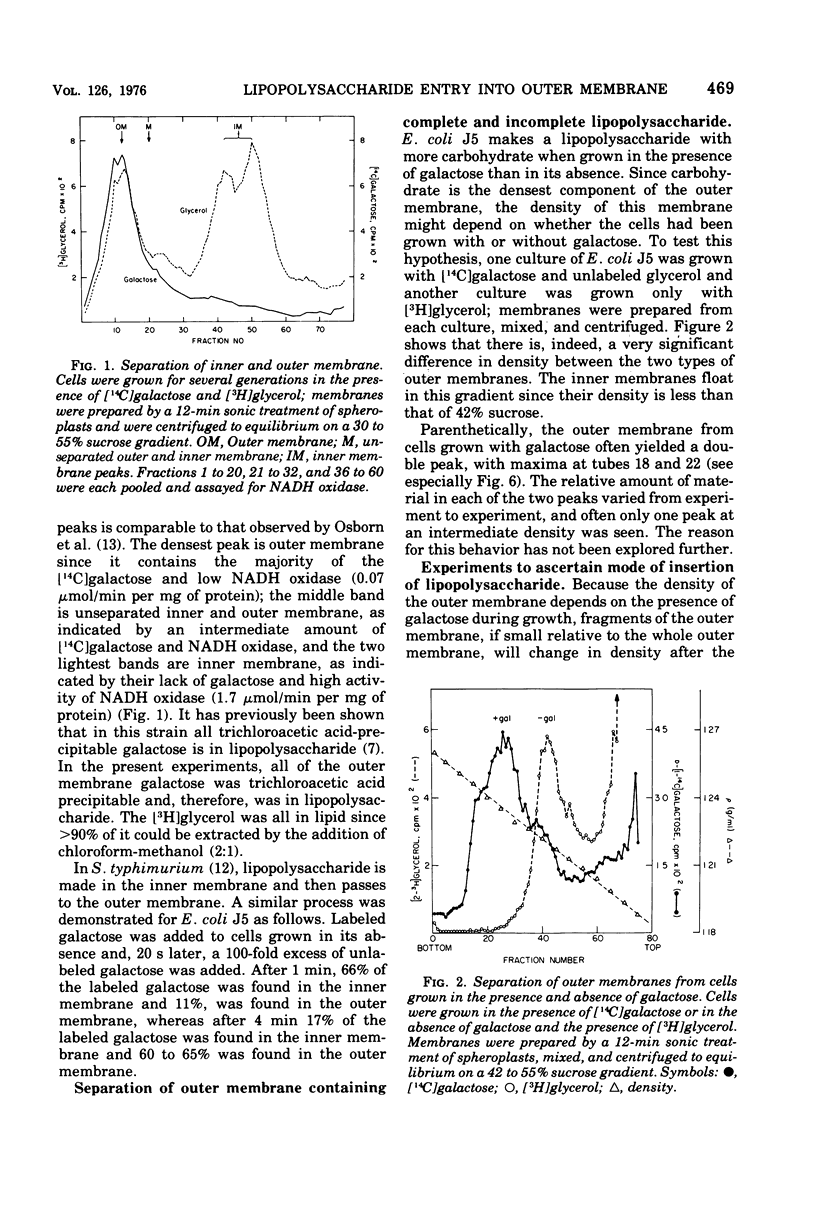
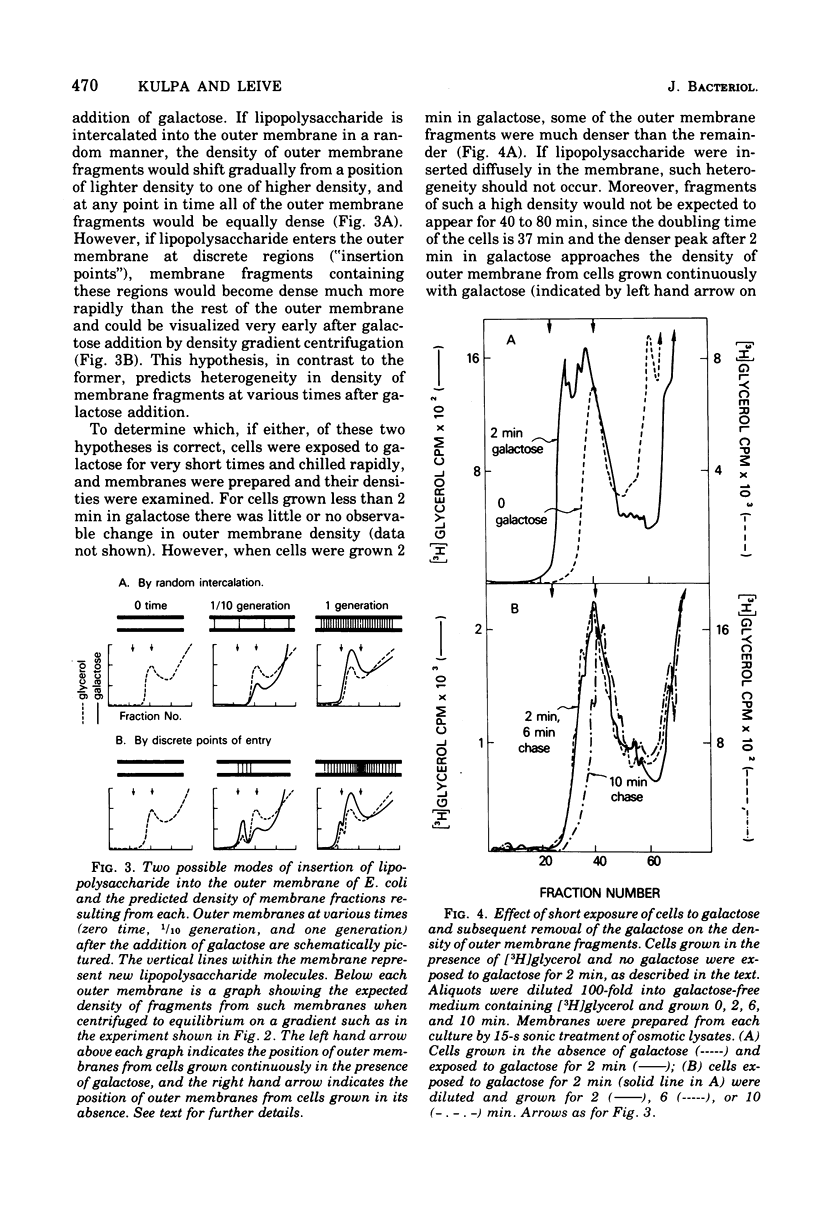
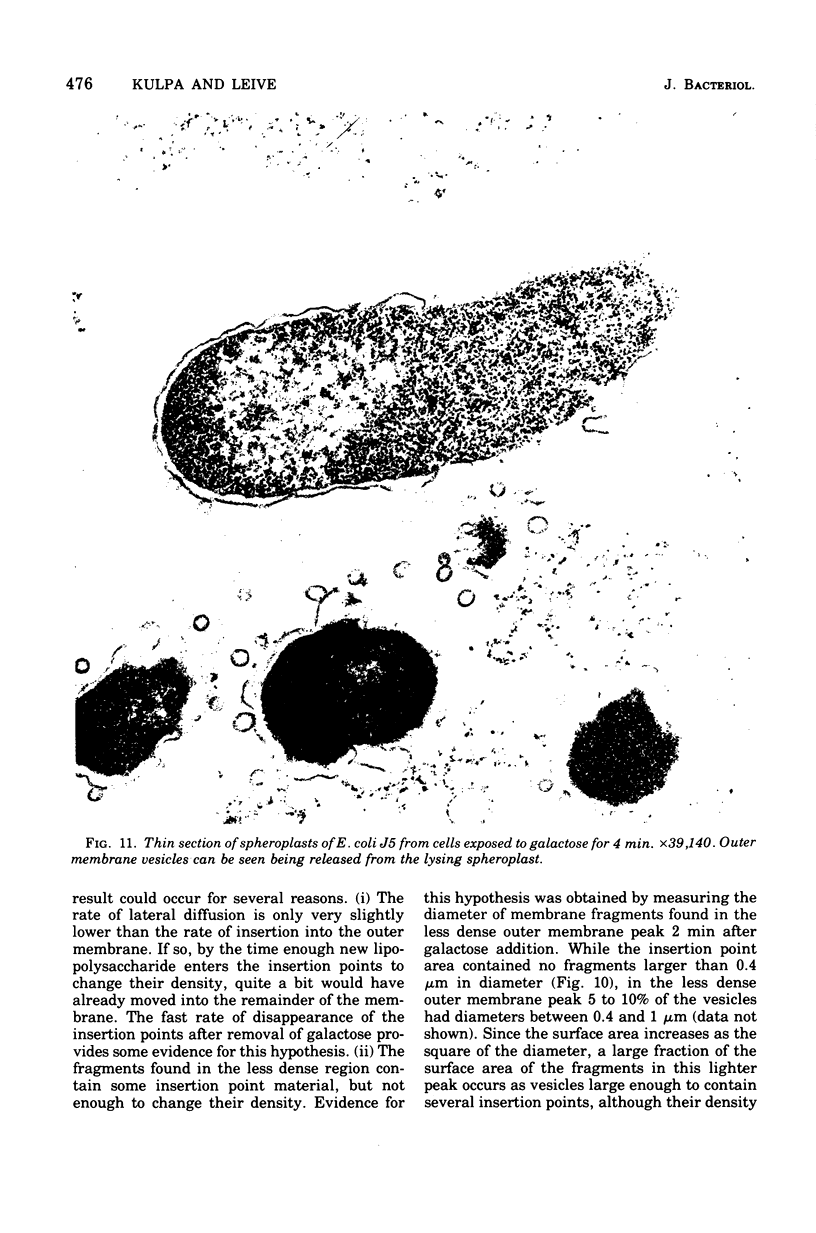
A mutant of Escherichia coli that lacks uridine 5'-diphosphate galactose-4-epimerase makes lipopolysaccharide with less carbohydrate than the parent, unless galactose is present during growth. Carbohydrate is dense, and the outer membrane, which contains lipopolysaccharide, was found to be denser when isolated from cells grown with galactose then when galactose was omitted. Cells given galactose after growth in its absence rapidly formed dense regions within the outer membrane that disappeared when galactose was removed. These results indicate that lipopolysaccharide enters the outer membrane nonrandomly at a minimum of 10 to 22 discrete "insertion points." Isopycnic centrifugation provides a method for isolating these regions.
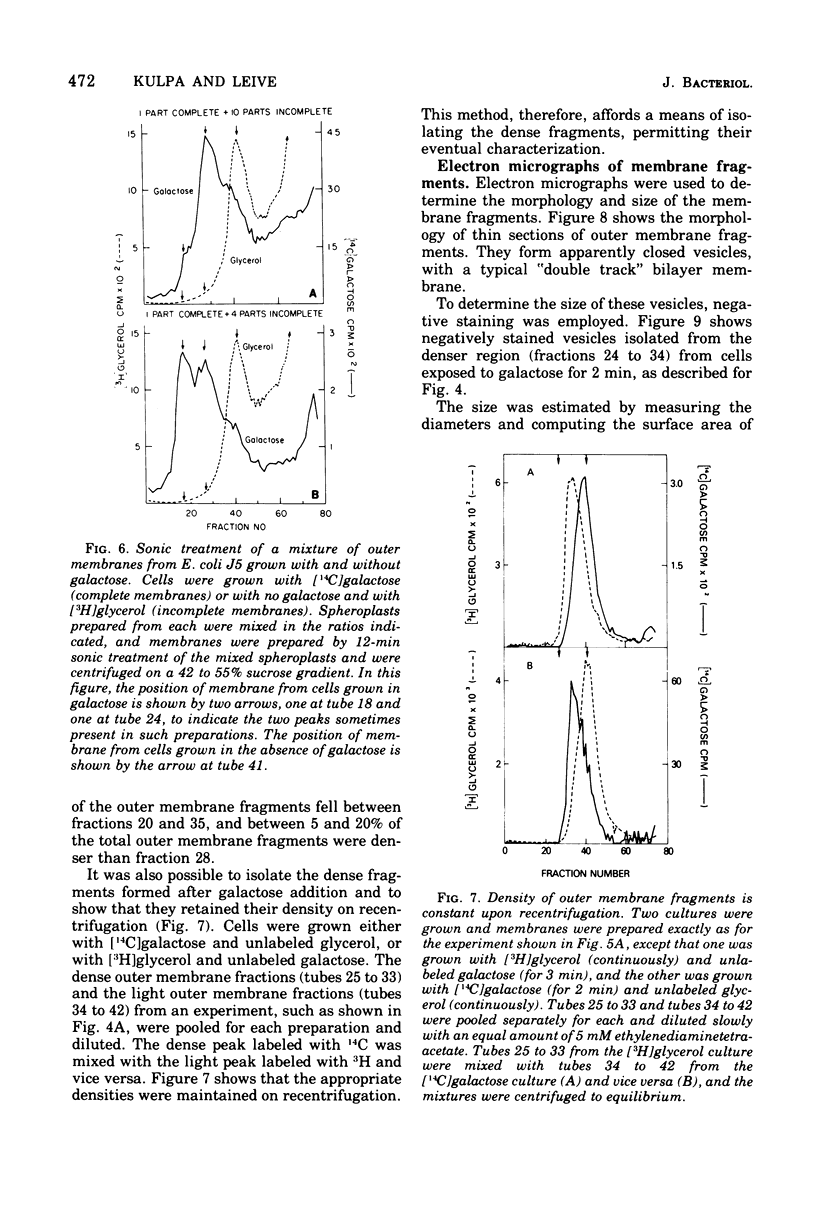
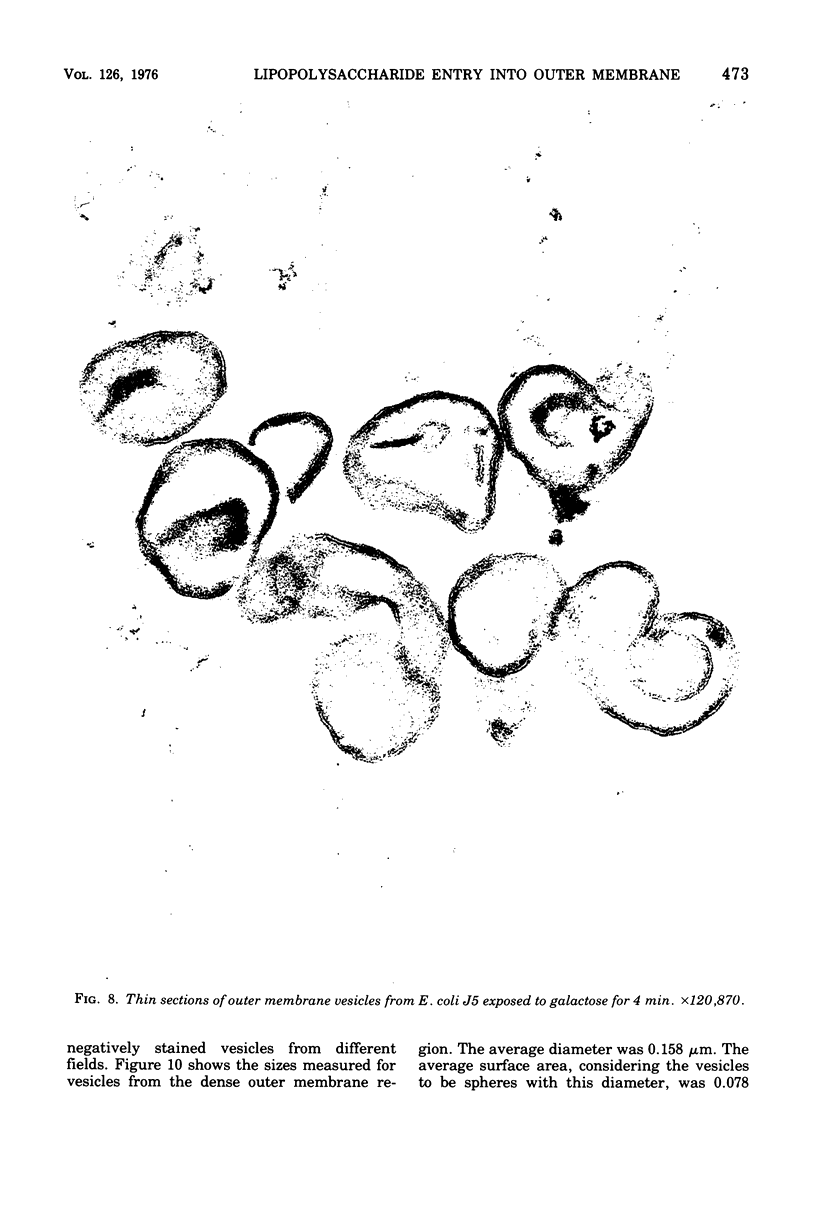
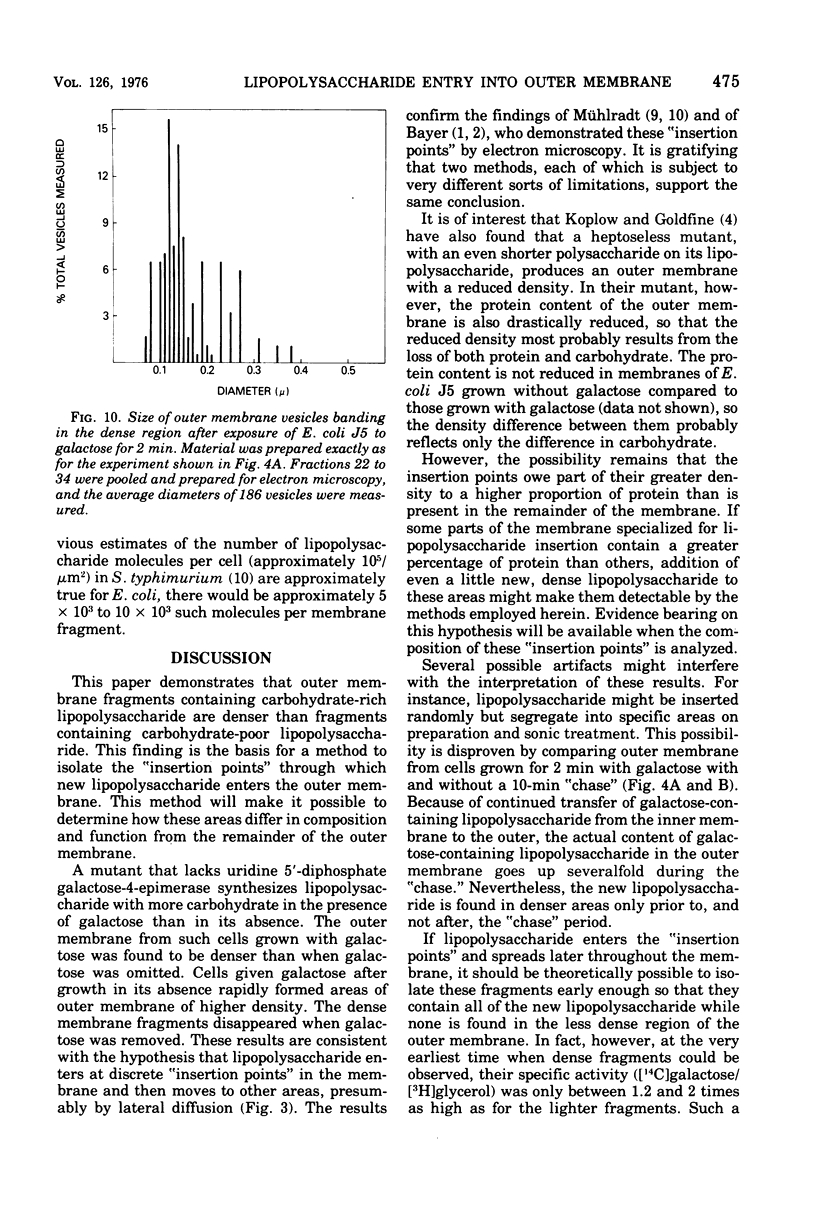
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Ultrastructure and organization of the bacterial envelope. Ann N Y Acad Sci. 1974 May 10;235(0):6–28. doi: 10.1111/j.1749-6632.1974.tb43254.x. [DOI] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Levy S. B., Leive L. An equilibrium between two fractions of lipopolysaccharide in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1435–1439. doi: 10.1073/pnas.61.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Lateral mobility and surface density of lipopolysaccharide in the outer membrane of Salmonella typhimurium. Eur J Biochem. 1974 Apr 16;43(3):533–539. doi: 10.1111/j.1432-1033.1974.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Outer membrane of salmonella. Sites of export of newly synthesised lipopolysaccharide on the bacterial surface. Eur J Biochem. 1973 Jun 15;35(3):471–481. doi: 10.1111/j.1432-1033.1973.tb02861.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Hybridization of membranes by sonic irradiation. Biochemistry. 1971 Aug 17;10(17):3309–3313. doi: 10.1021/bi00793a023. [DOI] [PubMed] [Google Scholar]