Abstract
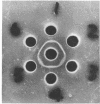
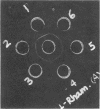
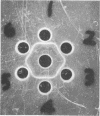
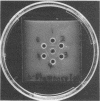
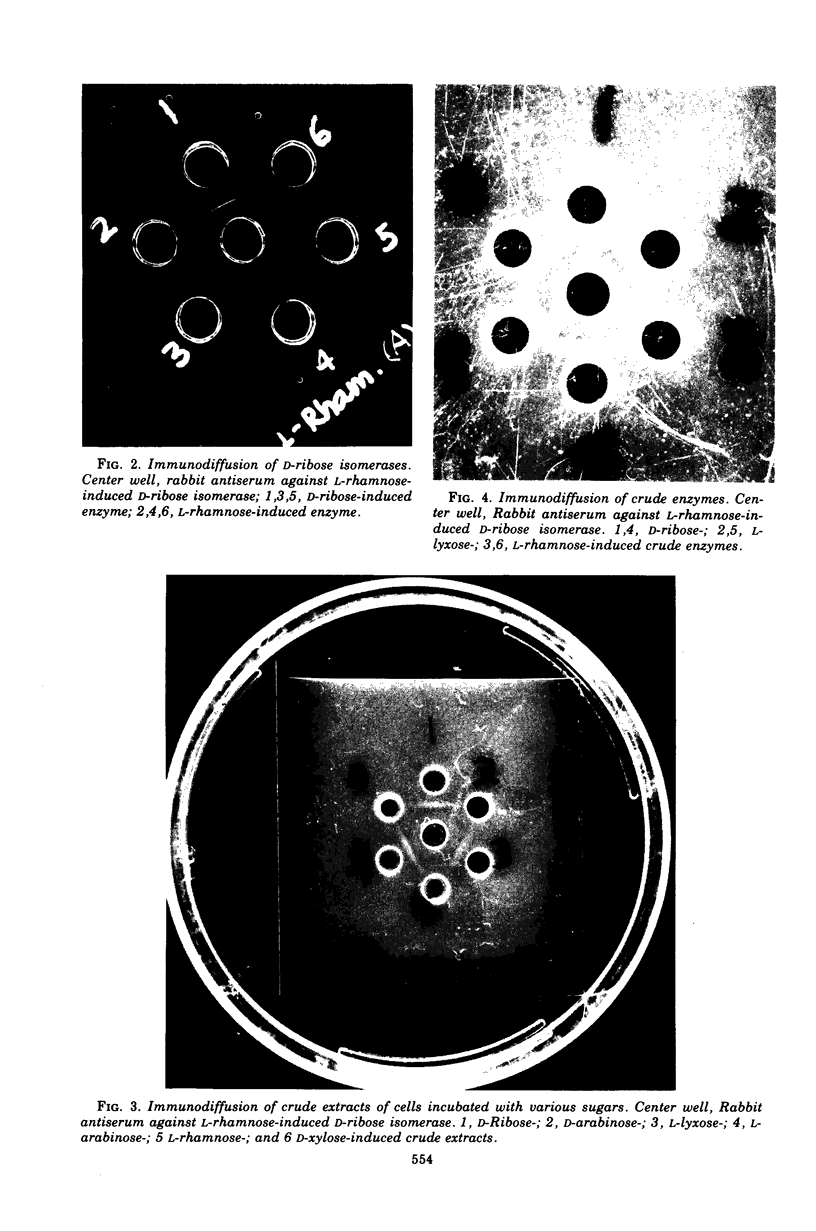
D-Ribose isomerase was purified and crystallized from cells of Mycobacterium smegmatis grown on either D-ribose or L-rhamnose. Isomerase activity for both of these sugars remained together throughout the purification. The isomerase from L-rhamnose-grown cells had the same chemical and physical properties as the enzyme isolated from D-ribose grown cells. In addition, immunological studies indicated that both activities were in the same protein since antisera prepared against either of the crystals cross-reacted with the other and gave lines of symmetry by the agar gel diffusion method.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Izumori K., Rees A. W., Elbein A. D. Purification, crystallization, and properties of D-ribose isomerase from Mycobacterium smegmatis. J Biol Chem. 1975 Oct 25;250(20):8085–8087. [PubMed] [Google Scholar]
- TAKAGI Y., SAWADA H. THE METABOLISM OF L-RHAMNOSE IN ESCHERICHIA COLI. I. L-RHAMNOSE ISOMERASE. Biochim Biophys Acta. 1964 Oct 23;92:10–17. doi: 10.1016/0926-6569(64)90263-9. [DOI] [PubMed] [Google Scholar]