Abstract
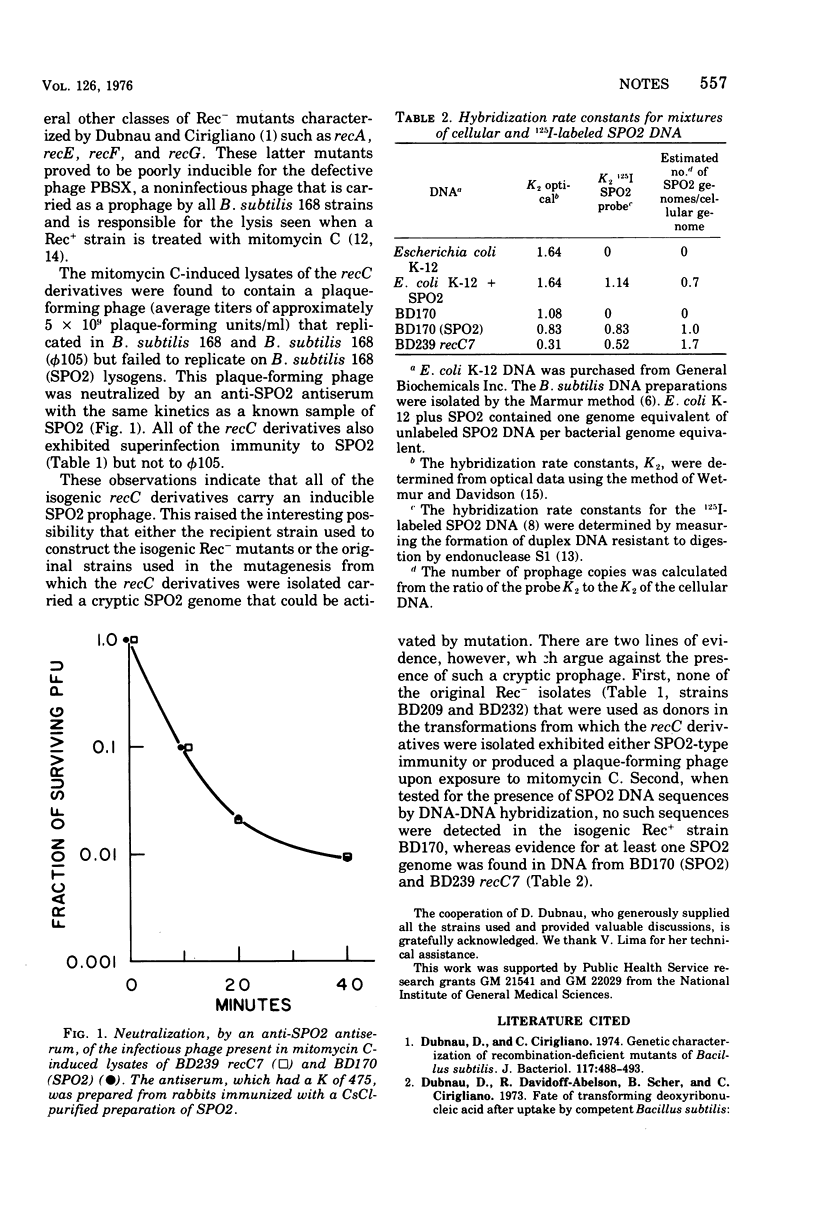
The recombination-defective phenotype associated with the recC genetic locus in Bacillus subtilis is not due to a chromosomal mutation at this site but rather to the presence of an integrated SPO2 prophage.
Full text
PDF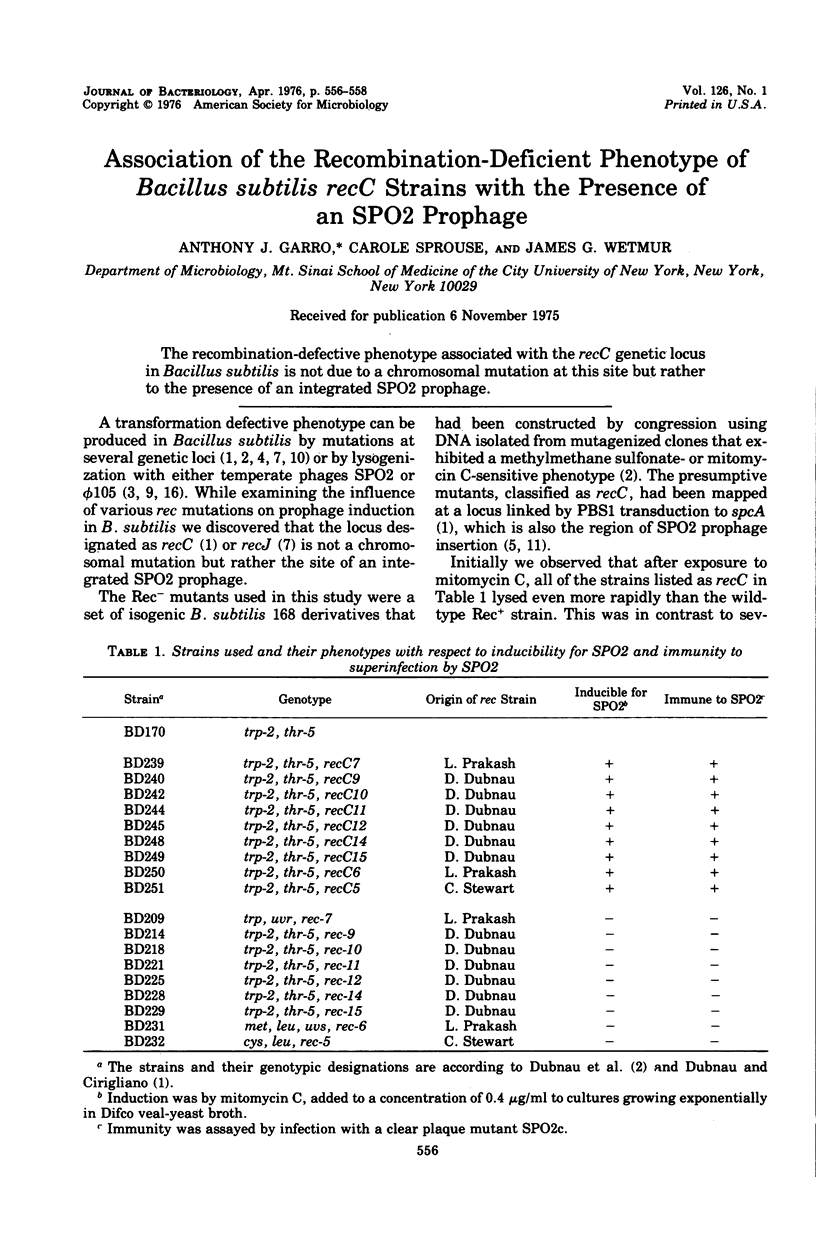

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubnau D., Cirigliano C. Genetic characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Feb;117(2):488–493. doi: 10.1128/jb.117.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. J., Law M. F. Relationship between lysogeny, spontaneous induction, and transformation efficiencies in Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1256–1259. doi: 10.1128/jb.120.3.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford N. Genetic analysis of rec mutants of Bacillus subtilis. Evidence for at least six linkage groups. Mol Gen Genet. 1974 Mar 27;129(3):269–274. doi: 10.1007/BF00267919. [DOI] [PubMed] [Google Scholar]
- Inselburg J. W., Eremenko-Volpe T., Greenwald L., Meadow W. L., Marmur J. Physical and genetic mapping of the SPO2 prophage on the chromosome of Bacillus subtilis 168. J Virol. 1969 Jun;3(6):627–628. doi: 10.1128/jvi.3.6.627-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G., Fortunato A., Ferrari E., Canosi U., Falaschi A., Polsinelli M. Genetic and enzymic studies on the recombination process in Bacillus subtilis. Mol Gen Genet. 1975;136(1):9–30. doi: 10.1007/BF00275445. [DOI] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. In vitro iodination of DNA. Maximizing iodination while minimizing degradation; use of buoyant density shifts for DNA-DNA hybrid isolation. Biochemistry. 1974 Dec 31;13(27):5467–5473. doi: 10.1021/bi00724a003. [DOI] [PubMed] [Google Scholar]
- Peterson A. M., Rutberg L. Linked transformation of bacterial and prophage markers in Bacillus subtilis 168 lysogenic for bacteriophage phi 105. J Bacteriol. 1969 Jun;98(3):874–877. doi: 10.1128/jb.98.3.874-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samojlenko I., Harford N., Mergeay M. Phenotypic properties of Bacillus subtilis mutants defective in recombination and repair functions. Mol Gen Genet. 1974 May 21;130(2):143–152. doi: 10.1007/BF00269085. [DOI] [PubMed] [Google Scholar]
- Smith I., Smith H. Location of the SPO2 attachment site and the bryamycin resistance marker on the Bacillus subtilis chromosome. J Bacteriol. 1973 Jun;114(3):1138–1142. doi: 10.1128/jb.114.3.1138-1142.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Thurm P., Garro A. J. Isolation and characterization of prophage mutants of the defective Bacillus subtilis bacteriophage PBSX. J Virol. 1975 Jul;16(1):184–191. doi: 10.1128/jvi.16.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


