Abstract
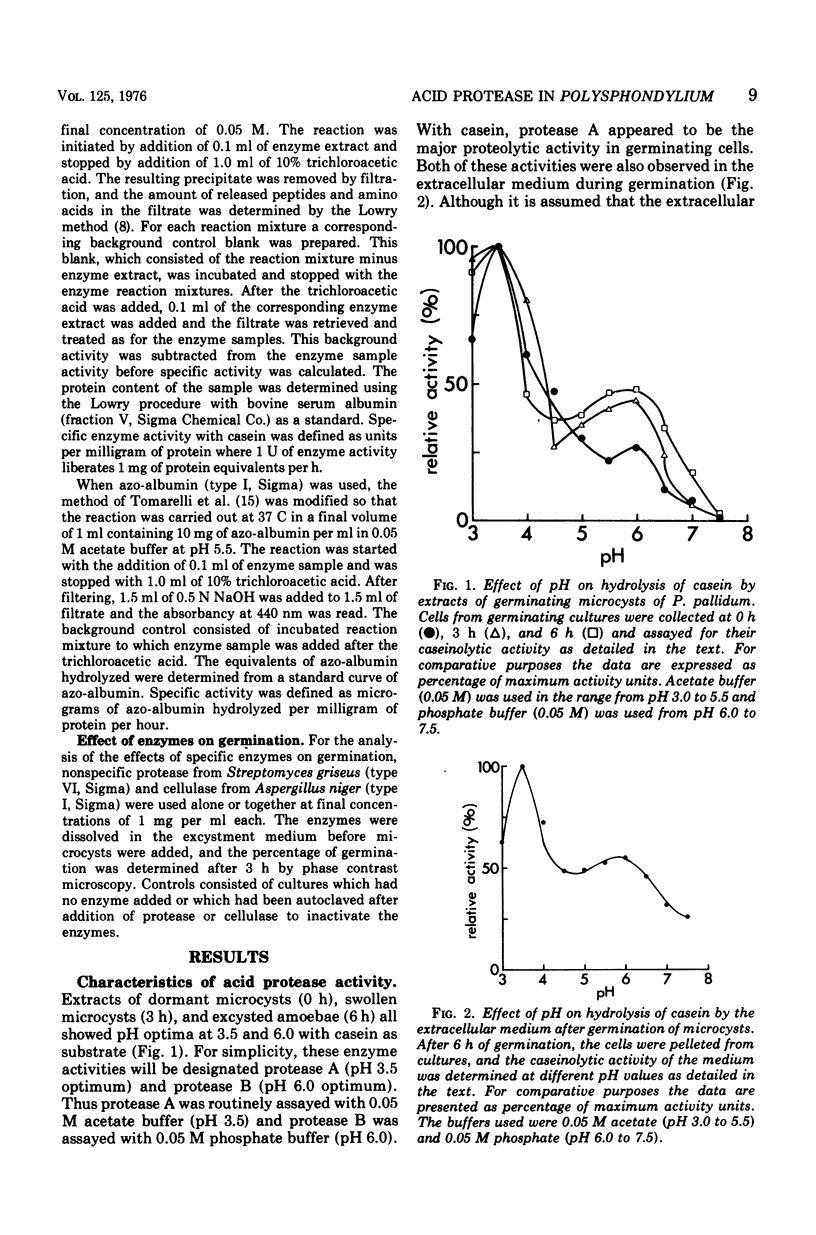
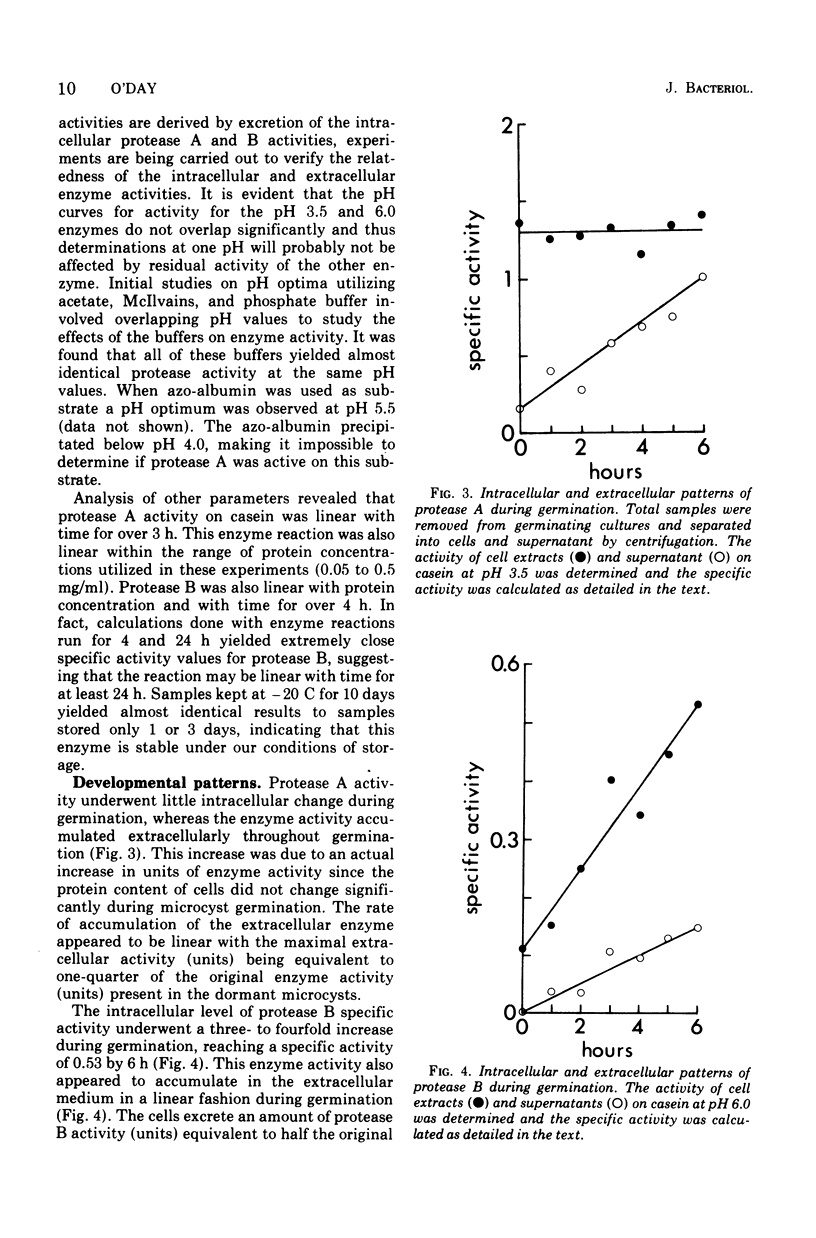
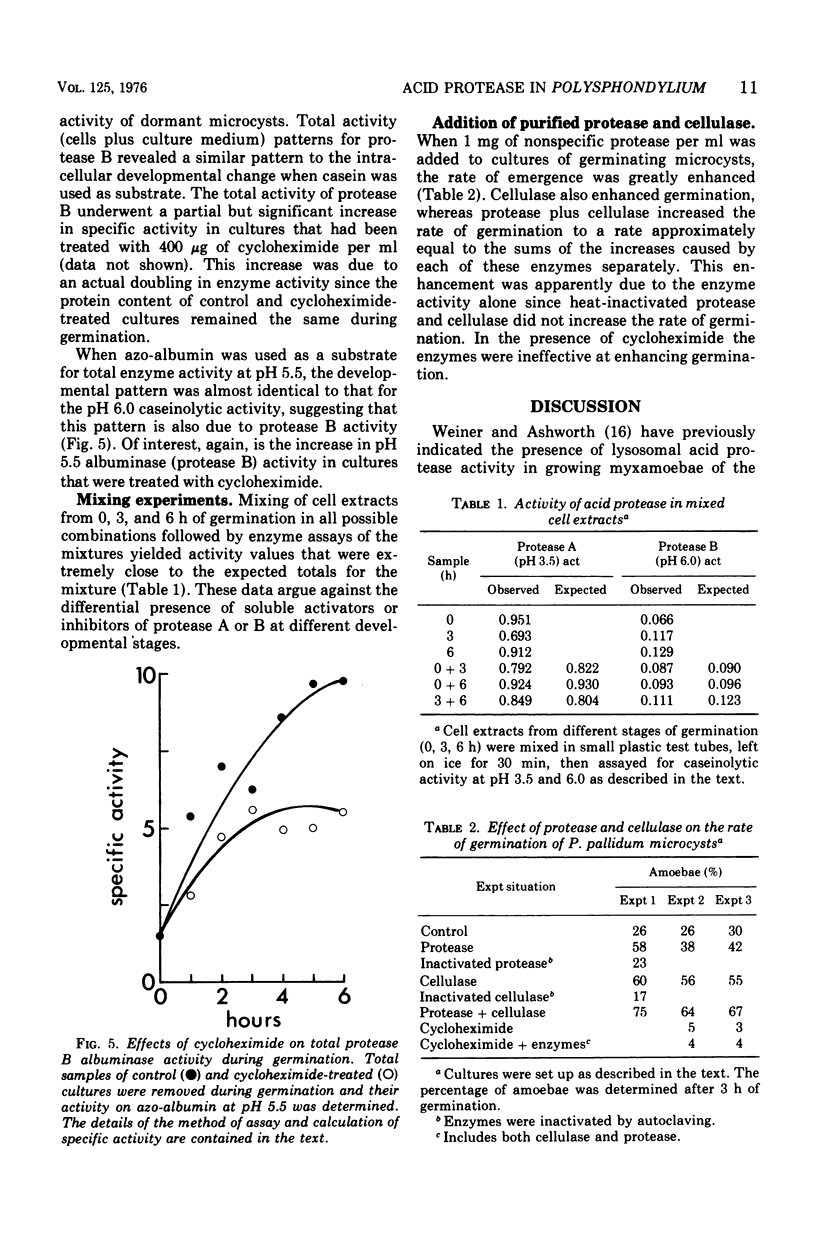
Extracts of dormant microcysts of Polysphondylium pallidum demonstrate pH optima for the hydrolysis of casein at 3.5 and 6.0. During germination the intracellular pH 6.0 caseinolytic specific activity does not change significantly. The pH 6.0 protease is also active on azo-albumin, revealing the same developmental pattern with this substrate. Both acid protease activities are excreted during the germination process. Addition of purified nonspecific protease to cultures speeds up germination, suggesting that the excreted protease may play a role in removal of the microcyst wall. When cycloheximide is added to cultures, complete germination (emergence) is stopped whereas the pH 6.0 protease activity still accumulates to between 50 and 60% of the maximum control activity. Although this suggests that post-translational controls might mediate the accumulation of a portion of the pH 6.0 protease increase, mixing and dilution experiments with cell extracts do not reveal the differential presence of soluble activators or inhibitors of this activity at different developmental stages. The presence of tightly bound enzyme-inhibitor complexes for protease B in dormant microcysts has not been ruled out and is currently under study.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Quance J. Enzyme synthesis in myxamoebae of the cellular slime mould Dictyostelium discoideum during growth in axenic culture. Biochem J. 1972 Feb;126(3):601–608. doi: 10.1042/bj1260601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Spore germination in strains of Dictyostelium discoideum and other members of the Dictyosteliaceae. J Bacteriol. 1968 Nov;96(5):1690–1695. doi: 10.1128/jb.96.5.1690-1695.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmes D. E., Kojima-Buddenhagen E. S., Hohl H. R. Structural and enzymatic analysis of the spore wall layers in Dictyostelium discoideum. J Ultrastruct Res. 1972 Dec;41(5):406–417. doi: 10.1016/s0022-5320(72)90047-0. [DOI] [PubMed] [Google Scholar]
- Hohl H. R., Miura-Santo L. Y., Cotter D. A. Ultrastructural changes during formation and germination of microcysts in Polysphondylium pallidum, a cellular slime mould. J Cell Sci. 1970 Jul;7(1):285–305. doi: 10.1242/jcs.7.1.285. [DOI] [PubMed] [Google Scholar]
- Killick K. A., Wright B. E. Trehalose synthesis during differentiation in Dictyostelium discoideum. IV. Secretion of trehalase and the in vitro expression of trehalose-6-phosphate synthetase activity. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1476–1481. doi: 10.1016/0006-291x(72)90880-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenney J. F., Matile P., Wiemken A., Schellenberg M., Meyer J. Activities and cellular localization of yeast proteases and their inhibitors. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1378–1383. doi: 10.1016/0006-291x(74)90350-7. [DOI] [PubMed] [Google Scholar]
- O'Day D. H. Alpha-mannosidase and microcyst differentiation in the cellular slime mold Polysphondylium pallidum. J Bacteriol. 1973 Jan;113(1):192–197. doi: 10.1128/jb.113.1.192-197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Day D. H. Intracellular and extracellular enzyme patterns during microcyst germination in the cellular slime mold Polysphondylium pallidum. Dev Biol. 1974 Feb;36(2):400–410. doi: 10.1016/0012-1606(74)90061-x. [DOI] [PubMed] [Google Scholar]
- O'Day D. R. Intracellular localization and extracellular release of certain lysosomal enzyme activities from amoebae of the cellular slime mould Polysphondylium pallidum. Cytobios. 1973 Jul-Aug;7(28):223–232. [PubMed] [Google Scholar]
- SUSSMAN M. Growth of the cellular slime mold Polysphondylium pallidum in a simple nutrient medium. Science. 1963 Jan 25;139(3552):338–338. doi: 10.1126/science.139.3552.338. [DOI] [PubMed] [Google Scholar]
- Toama M. A., Raper K. B. Microcysts of the cellular slime mold Polysphondylium pallidum. I. Factors influencing microcyst formation. J Bacteriol. 1967 Oct;94(4):1143–1149. doi: 10.1128/jb.94.4.1143-1149.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toama M. A., Raper K. B. Microcysts of the cellular slime mold Polysphondylium pallidum. II. Chemistry of the microcyst walls. J Bacteriol. 1967 Oct;94(4):1150–1153. doi: 10.1128/jb.94.4.1150-1153.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener E., Ashworth J. M. The isolation and characterization of lysosomal particles from myxamoebae of the cellular slime mould Dictyostelium discoideum. Biochem J. 1970 Jul;118(3):505–512. doi: 10.1042/bj1180505. [DOI] [PMC free article] [PubMed] [Google Scholar]


