Abstract
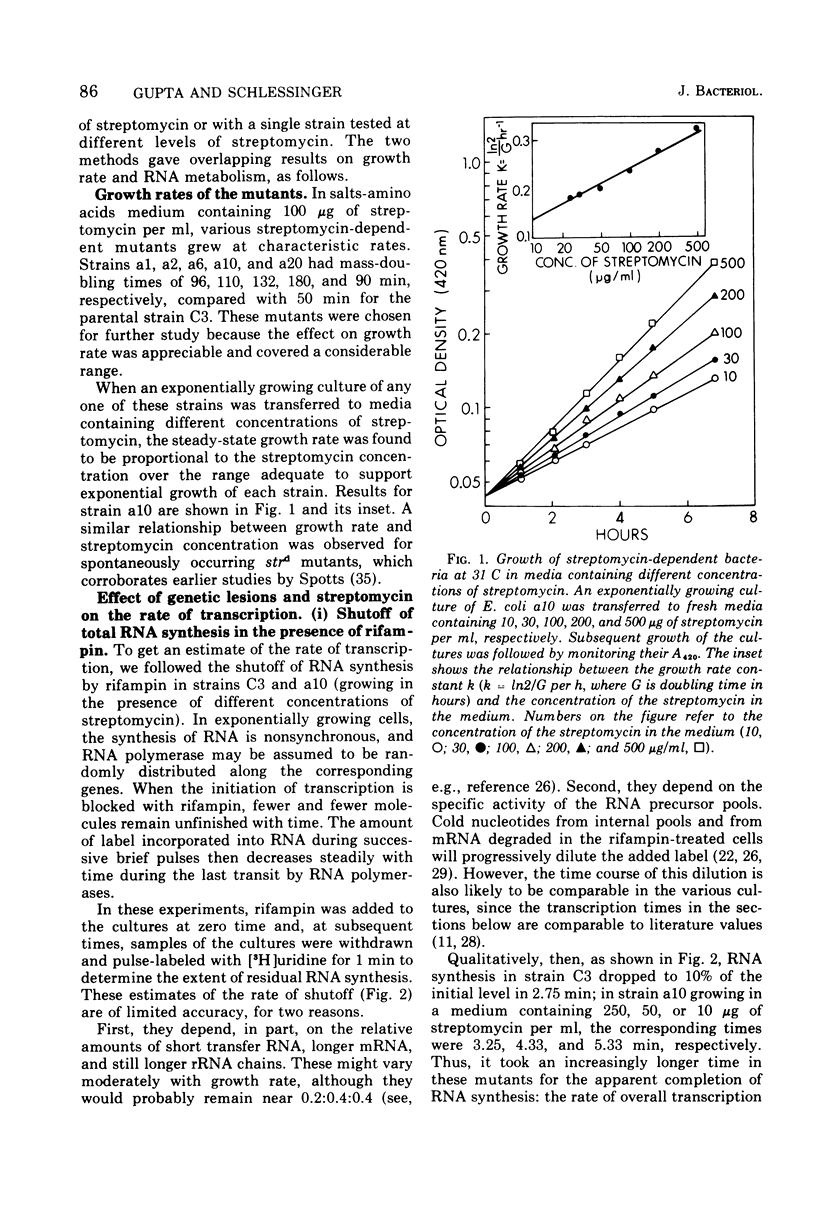
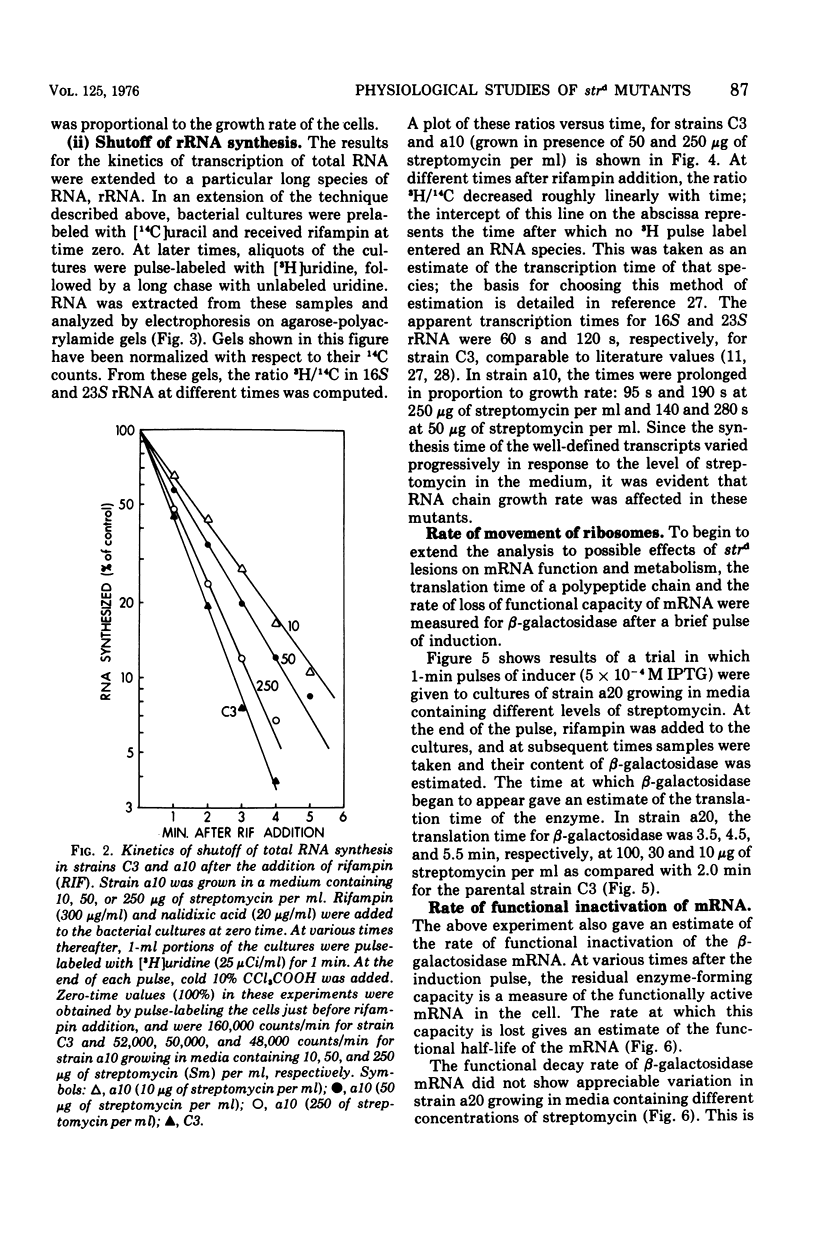
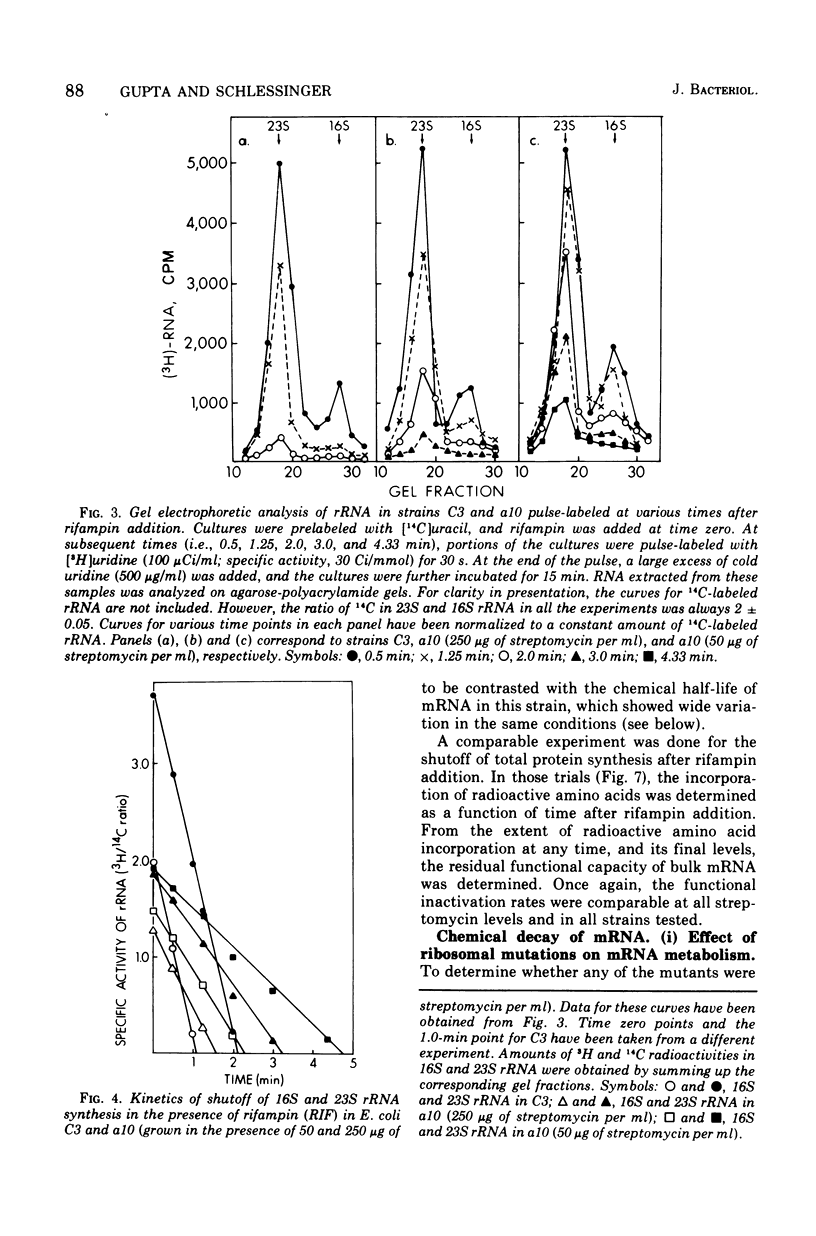
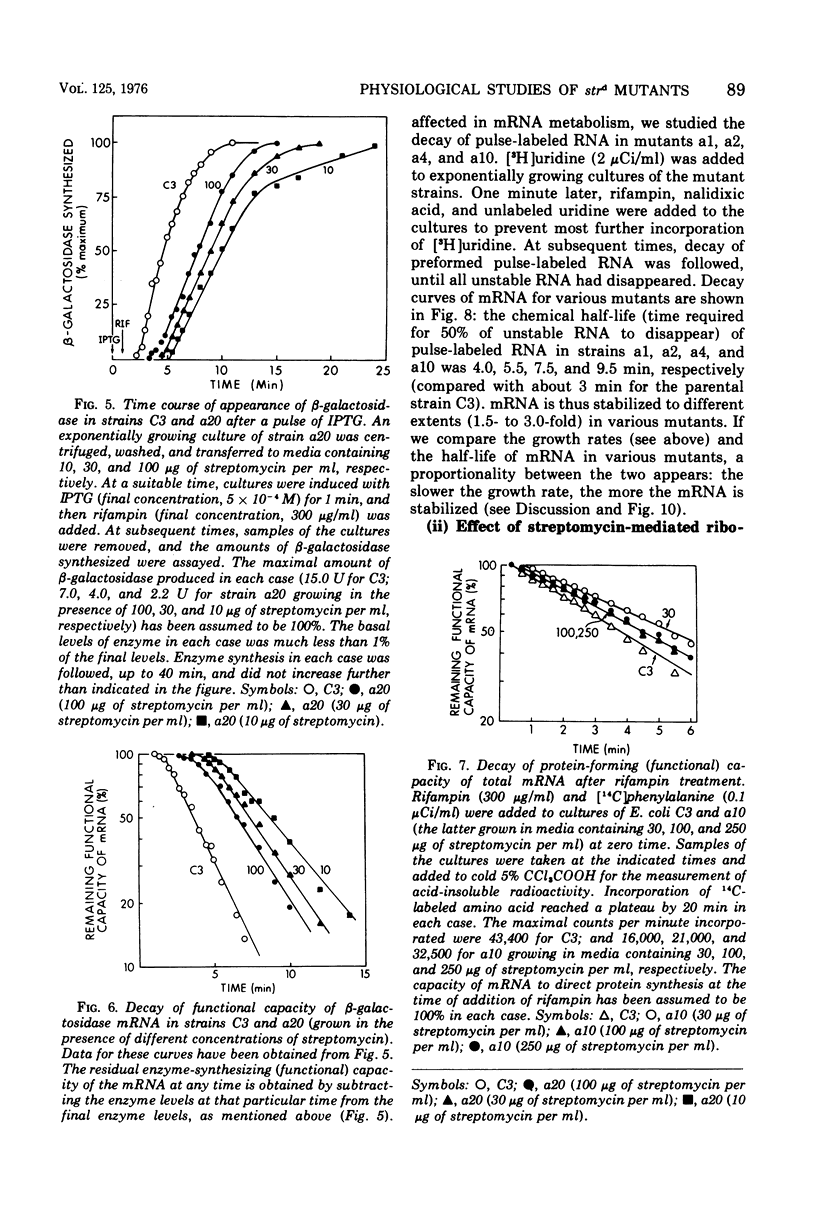
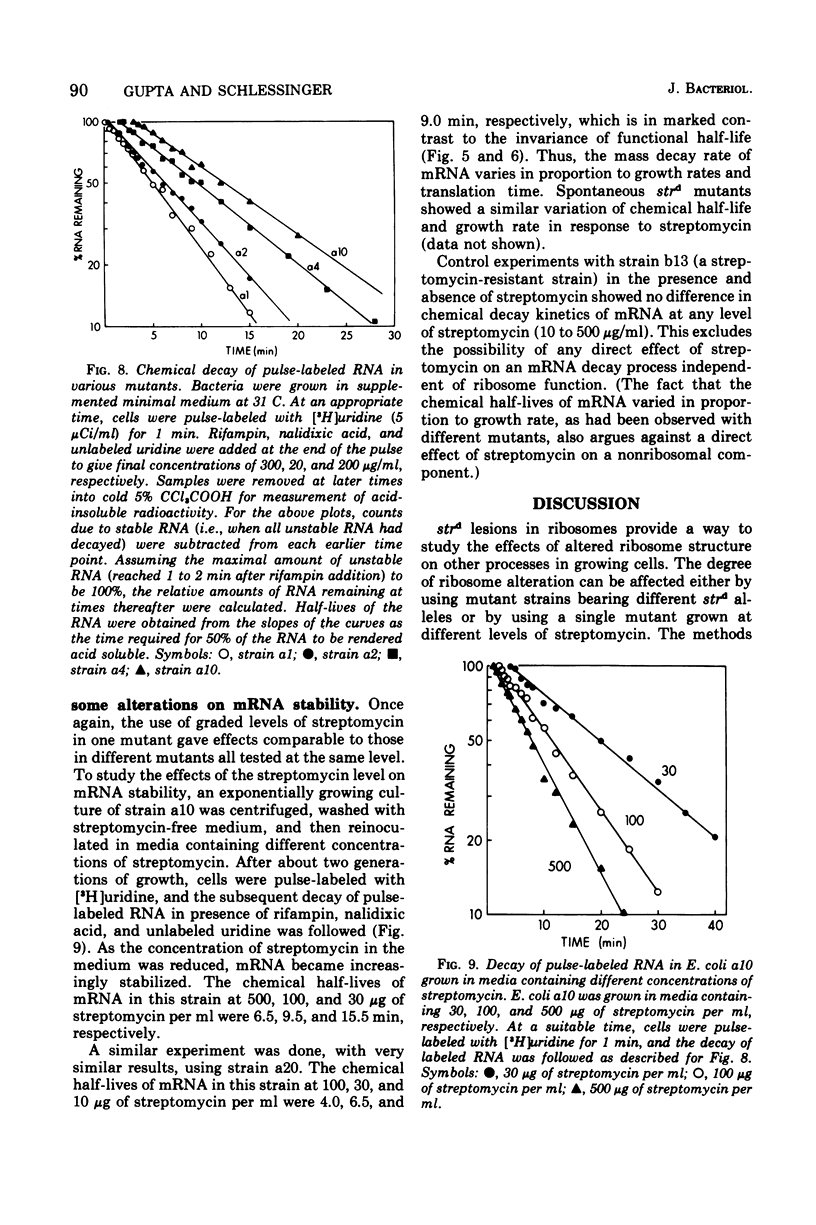
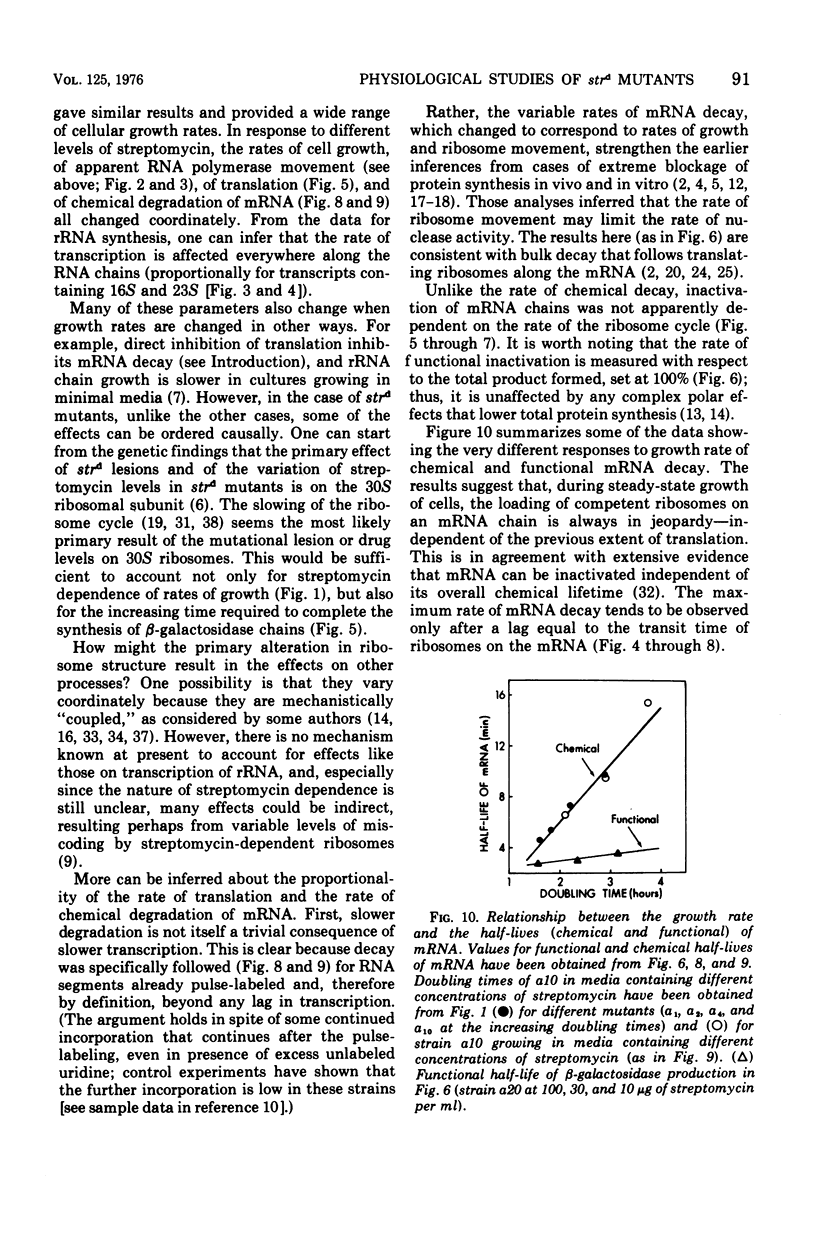
The growth rates of streptomycin-dependent mutants varied in proportion to the level of streptomycin supplied; growth also varied characteristically from one dependent strain to another at a given streptomycin concentration. When cells growing at different rates (over a threefold range) were treated with rifampin, direct proportionality was observed for three parameters: (i) the rates of shutoff of transcription of total ribonucleic acid (RNA) and ribosomal RNA, as measured by pulse labeling at later times; (ii) the translation time for molecules of beta-galactosidase; and (iii) the rate of chemical degradation of messenger RNA. In contrast, the rate of functional inactivation of both total and beta-galactosidase messenger RNA was about the same at all growth rates. None of the variations of growth or other parameters were observed in an otherwise isogenic streptomycin-resistant strain treated with streptomycin. Since the mutational change in strd mutants and the site of action of streptomycin are in the 30S ribosomal subunits, it is suggested that the rate of ribosome function is set by the dependent lesion (and the level of streptomycin). One possibility is that the other correlated effects are mechanistically "coupled" to ribosome function, but the apparent coupling could also be an indirect result of differential effects of streptomycin on variables such as ribosomal miscoding and nucleotide pool size. However, since the rate of functional inactivation of messenger RNA is constant even when the RNA is broken down two- to fourfold more slowly, translation yield tends to be proportional to the growth rate of the dependent strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. M., Maaloe O. The effects of fusidic acid on growth, ribosome synthesis and RNA metabolism in Escherichia coli. J Mol Biol. 1974 Dec 15;90(3):541–561. doi: 10.1016/0022-2836(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Craig E., Cremer K., Schlessinger D. Metabolism of T4 messenger RNA, host messenger RNA and ribosomal RNA in T4-infected Escherichia coli B. J Mol Biol. 1972 Nov 28;71(3):701–715. doi: 10.1016/s0022-2836(72)80033-0. [DOI] [PubMed] [Google Scholar]
- Craig E. Synthesis of Specific, Stabilized Messenger RNA When Translocation Is Blocked in ESCHERICHIA COLI. Genetics. 1972 Feb;70(2):331–336. doi: 10.1093/genetics/70.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K., Schlessinger D. Ca2+ ions inhibit messenger ribonucleic acid degradation, but permit messenger ribonucleic acid transcription and translation in deoxyribonucleic acid-coupled systems from Escherichia coli. J Biol Chem. 1974 Aug 10;249(15):4730–4736. [PubMed] [Google Scholar]
- Cremer K., Silengo L., Schlessinger D. Polypeptide formation and polyribosomes in Escherichia coli treated with chloramphenicol. J Bacteriol. 1974 May;118(2):582–589. doi: 10.1128/jb.118.2.582-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Nomura M. The genetics of bacterial ribosomes. Annu Rev Genet. 1972;6:203–234. doi: 10.1146/annurev.ge.06.120172.001223. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Regulation of ribonucleic acid synthesis in Escherichia coli B-r: an analysis of a shift-up. 1. Ribosomal RNA chain growth rates. J Mol Biol. 1973 Mar 25;75(1):145–159. doi: 10.1016/0022-2836(73)90535-4. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Schlessinger D. Differential modes of chemical decay for early and late lambda messenger RNA. J Mol Biol. 1975 Feb 25;92(2):311–318. doi: 10.1016/0022-2836(75)90230-2. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Singh U. N. Initiation of transcription and the sequential synthesis of 16-S and 23-S ribosomal RNA in Escherichia coli. Biochim Biophys Acta. 1972 Sep 14;277(3):567–575. doi: 10.1016/0005-2787(72)90100-1. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Apirion D., Schlessinger D. Polyribosome metabolism in Escherichia coli treated with chloramphenicol, neomycin, spectinomycin or tetracycline. J Mol Biol. 1969 Oct 28;45(2):205–220. doi: 10.1016/0022-2836(69)90100-4. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Bennett P. M., von Meyenburg K. Intracistronic polarity during dissociation of translation from transcription in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):589–604. doi: 10.1016/0022-2836(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Kano Y. Inhibition of transcription of the tryptophan operon in Escherichia coli by a block in initiation of translation. Nat New Biol. 1971 Aug 11;232(2):169–173. doi: 10.1038/newbio232169a0. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Schlessinger D. Bearing of some recent results on the mechanisms of polarity and messenger RNA stability. Mol Gen Genet. 1974;135(1):29–38. doi: 10.1007/BF00433898. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Apirion D., Schlessinger D. Mechanism of action of streptomycin in E. coli: interruption of the ribosome cycle at the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1968 Jul;60(3):873–880. doi: 10.1073/pnas.60.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Schlessinger D., Kuwano M. Initiation of ribosome-dependent breakdown of T4-specific messenger RNA. J Mol Biol. 1971 Sep 28;60(3):441–452. doi: 10.1016/0022-2836(71)90180-x. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Turco E. Ribonuclease activity in Escherichia coli polyribosomes. Eur J Biochem. 1973 Oct 18;38(3):507–515. doi: 10.1111/j.1432-1033.1973.tb03086.x. [DOI] [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Morikawa N., Imamoto F. Degradation of tryptophan messenger. On the degradation of messenger RNA for the tryptophan operon in Escherichia coli. Nature. 1969 Jul 5;223(5201):37–40. doi: 10.1038/223037a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R., Baker R. F., Yanofsky C. Direction of in vivo degradation of tryptophan messenger RNA--a correction. Nature. 1969 Jul 5;223(5201):40–43. doi: 10.1038/223040a0. [DOI] [PubMed] [Google Scholar]
- Mowbray S. L., Nierlich D. P. Regulation of RNA synthesis in Escherichia coli during a shift-up transition. Biochim Biophys Acta. 1975 Jun 2;395(1):91–107. doi: 10.1016/0005-2787(75)90237-3. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Birenbaum M., Schlessinger D. 30 S pre-ribosomal RNA of Escherichia coli:primary and secondary processing. Biochim Biophys Acta. 1975 Jul 23;395(4):478–489. doi: 10.1016/0005-2787(75)90071-4. [DOI] [PubMed] [Google Scholar]
- Pace N. R. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. doi: 10.1128/br.37.4.562-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushok C., Kennell D. Residual polarity and transcription-translation coupling during recovery from chloramphenicol or fusidic acid. J Bacteriol. 1974 Feb;117(2):631–640. doi: 10.1128/jb.117.2.631-640.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOTTS C. R. Physiological and biochemical studies on streptomycin dependence in Escherichia coli. J Gen Microbiol. 1962 Jun;28:347–365. doi: 10.1099/00221287-28-2-347. [DOI] [PubMed] [Google Scholar]
- SPOTTS C. R., STANIER R. Y. Mechanism of streptomycin action on bacteria: a unitary hypothesis. Nature. 1961 Nov 18;192:633–637. doi: 10.1038/192633a0. [DOI] [PubMed] [Google Scholar]
- Schwartz T., Craig E., Kennell D. Inactivation and degradation of messenger ribnucleic acid from the lactose operon of Escherichia coli. J Mol Biol. 1970 Dec 14;54(2):299–311. doi: 10.1016/0022-2836(70)90431-6. [DOI] [PubMed] [Google Scholar]
- Singh U. N., Gupta R. S. Polyribosomes and unstable messenger RNA. II. Some further implications of the tape theory of protein synthesis. J Theor Biol. 1971 Mar;30(3):603–619. doi: 10.1016/0022-5193(71)90013-0. [DOI] [PubMed] [Google Scholar]
- Singh U. N. Polyribosomes and unstable messenger RNA: a stochastic model of protein synthesis. J Theor Biol. 1969 Dec;25(3):444–460. doi: 10.1016/s0022-5193(69)80032-9. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Tai P. C., Davis B. D. Effect of streptomycin on the response of Escherichia coli ribosomes to the dissociation factor. J Mol Biol. 1973 Apr 5;75(2):391–400. doi: 10.1016/0022-2836(73)90029-6. [DOI] [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. The rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Mar 10;244(5):1128–1136. [PubMed] [Google Scholar]


