Abstract
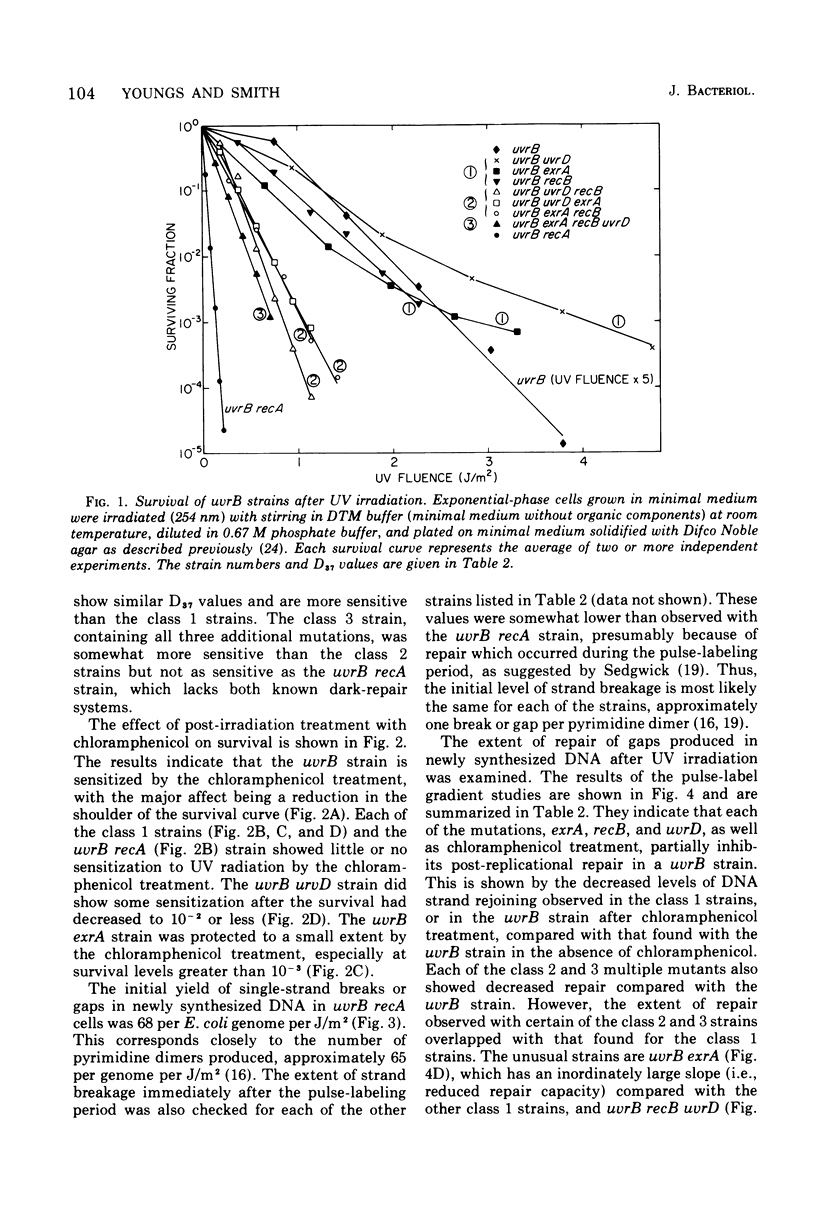
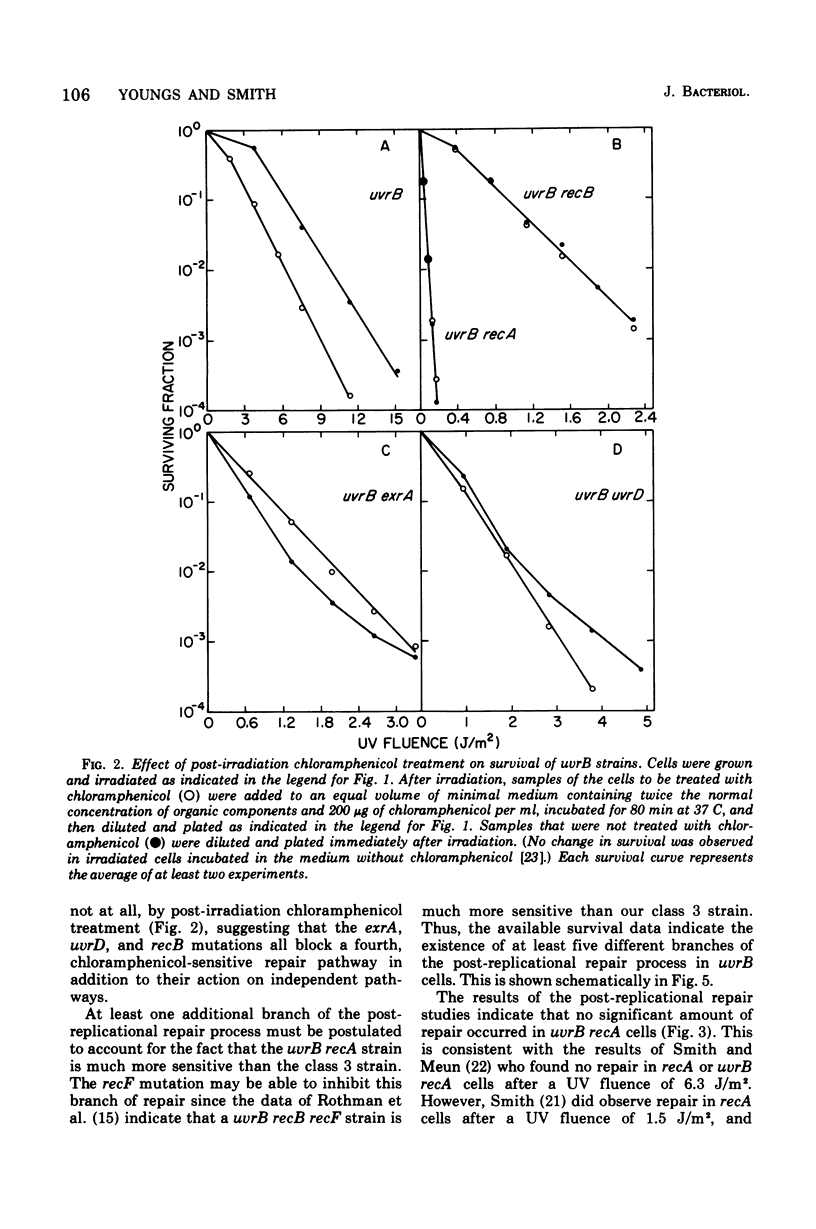
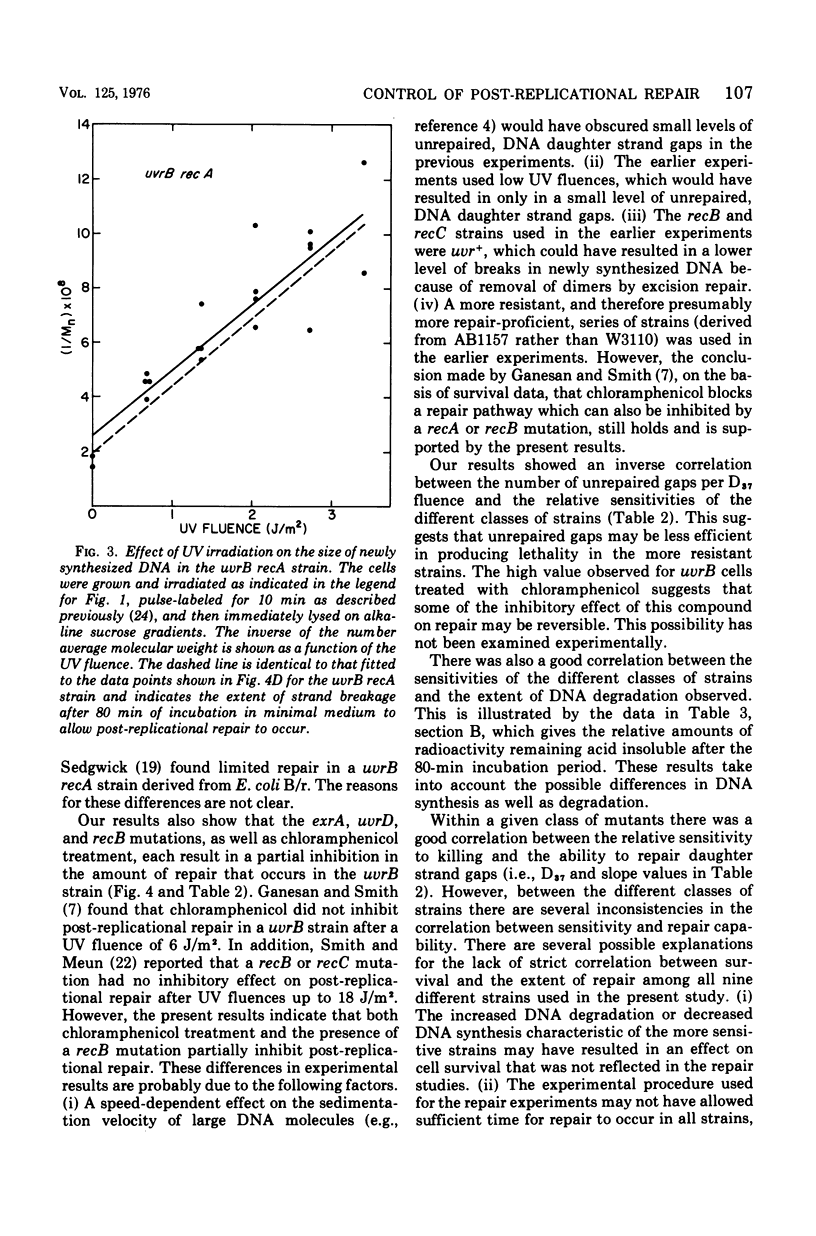
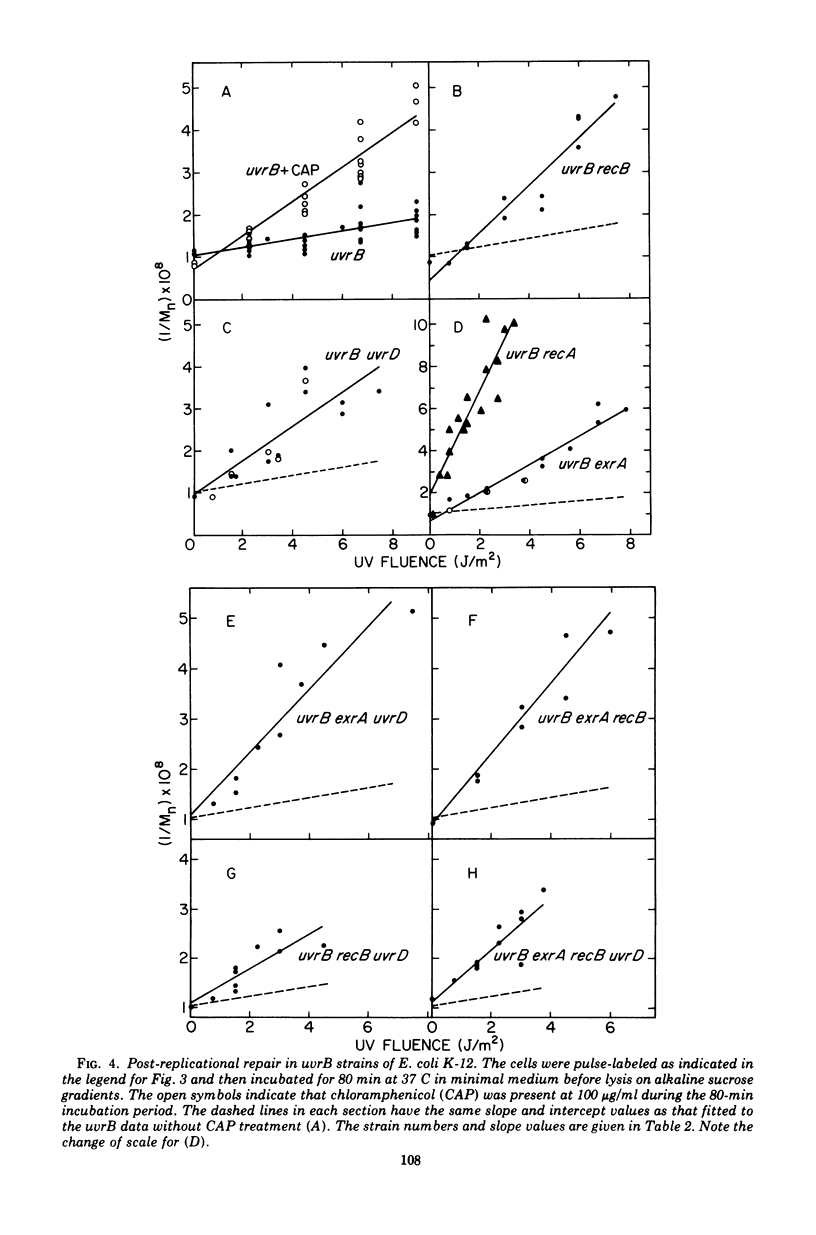
The effect of the recA, uvrD, exrA, and recB mutations and of post-irradiation treatment with chloramphenicol on the survival and post-replication repair after ultraviolet irradiation of uvrB strains of Escherichia coli K-12 was examined. Each of these mutations or treatments was found to decrease survival and the extent of repair. The interactions of the inhibitory effects of the uvrD, exaA, and recB mutations and chloramphenicol treatment were determined by examining the survival and repair characteristics of the several multiple mutants. The survival results suggest that the post-replication repair process in uvrB strains may be subdivided into at least five different branches. These include three branches that are blocked by the exrA, recB, or uvrD mutation, a fourth branch that is blocked by any of these mutations and is also sensitive to chloramphenicol treatment, and at least one additional branch that is not sensitive to either of these mutations or to chloramphenicol treatment. The extent of post-replicational repair observed with each of the strains is in general agreement with the pathways postulated on the basis of the survival data, although there are several apparent exceptions to this correlation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Interactions among genes controlling sensitivity to radiation and alkylation in yeast. Mol Gen Genet. 1973 Sep 12;125(3):197–216. doi: 10.1007/BF00270743. [DOI] [PubMed] [Google Scholar]
- Chia D., Schumaker V. N. A rotor speed dependent crossover in sedimentation velocities of DNA's of different sizes. Biochem Biophys Res Commun. 1974 Jan;56(1):241–246. doi: 10.1016/s0006-291x(74)80340-2. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Dark-recovery processes in Escherichia coli irradiated with ultraviolet light. 3. Effect of rec mutations on recovery of excision-deficient mutants of Escherichia coli K-12. J Bacteriol. 1970 May;102(2):404–410. doi: 10.1128/jb.102.2.404-410.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan A. K., Smith K. C. Requirement for protein synthesis in rec-dependent repair of deoxyribonucleic acid in Escherichia coli after ultraviolet or X irradiation. J Bacteriol. 1972 Aug;111(2):575–585. doi: 10.1128/jb.111.2.575-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Jagger J., Fossum T., McCaul S. Ultraviolet irradiation of suspensions of micro-organisms: possible errors involved in the estimation of average fluence per cell. Photochem Photobiol. 1975 May;21(5):379–382. doi: 10.1111/j.1751-1097.1975.tb06690.x. [DOI] [PubMed] [Google Scholar]
- MOROWITZ H. J. Absorption effects in volume irradiation of microorganisms. Science. 1950 Mar 3;111(2879):229–229. doi: 10.1126/science.111.2879.229-a. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Rothman R. H., Kato T., Clark A. J. The beginning of an investigation of the role of recF in the pathways of metabolism of ultraviolet-irradiated DNA in Escherichia coli. Basic Life Sci. 1975;5A:283–291. doi: 10.1007/978-1-4684-2895-7_37. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Genetic and kinetic evidence for different types of postreplication repair in Escherichia coli B. J Bacteriol. 1975 Jul;123(1):154–161. doi: 10.1128/jb.123.1.154-161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G. Inducible error-prone repair in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2753–2757. doi: 10.1073/pnas.72.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Van der Schueren E., Youngs D. A., Smith K. C. Sensitization of ultraviolet-irradiated Escherichia coli K-12 by different agars: inhibition of A rec and exr gene-dependent branch of the uvr gene-dependent excision-repair process. Photochem Photobiol. 1974 Jul;20(1):9–13. doi: 10.1111/j.1751-1097.1974.tb06541.x. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Evidence for the control by exrA and polA genes of two branches of the uvr gene-dependent excision repair pathway in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):175–182. doi: 10.1128/jb.116.1.175-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



