Abstract
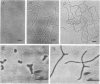
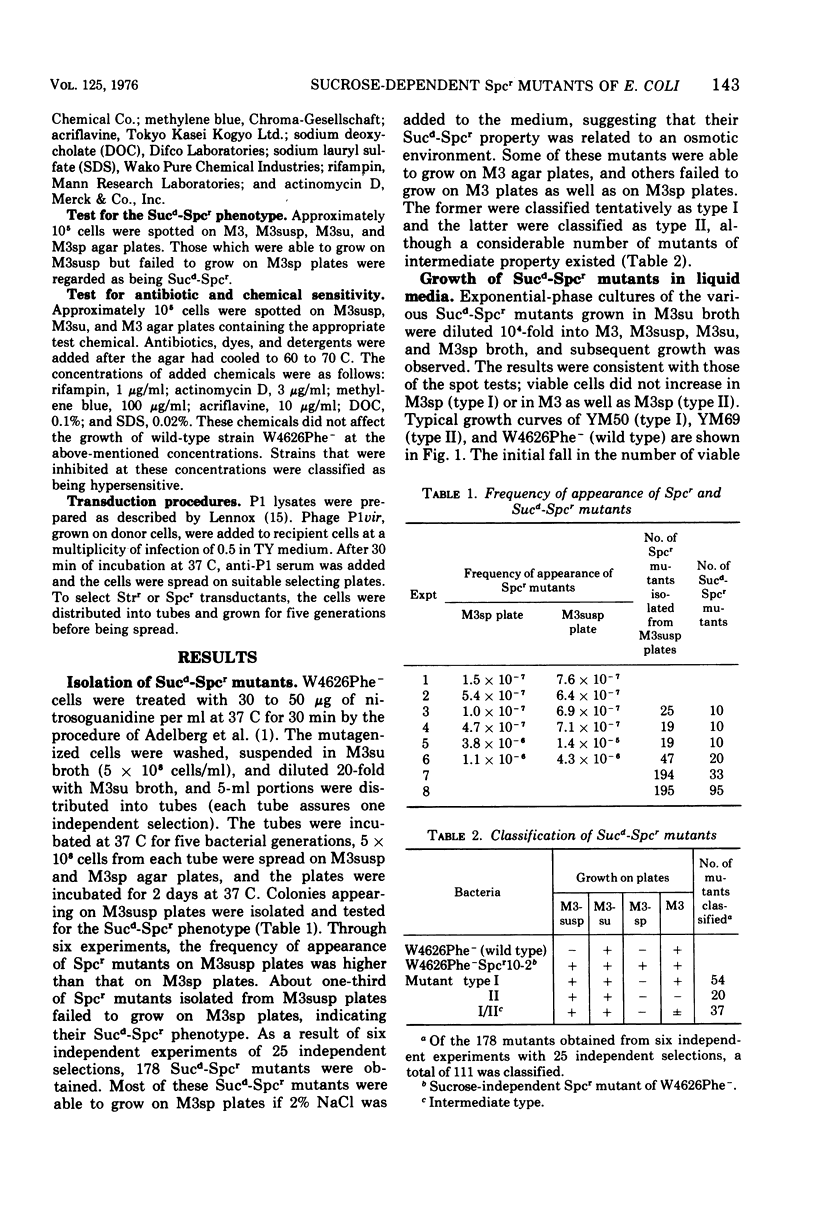
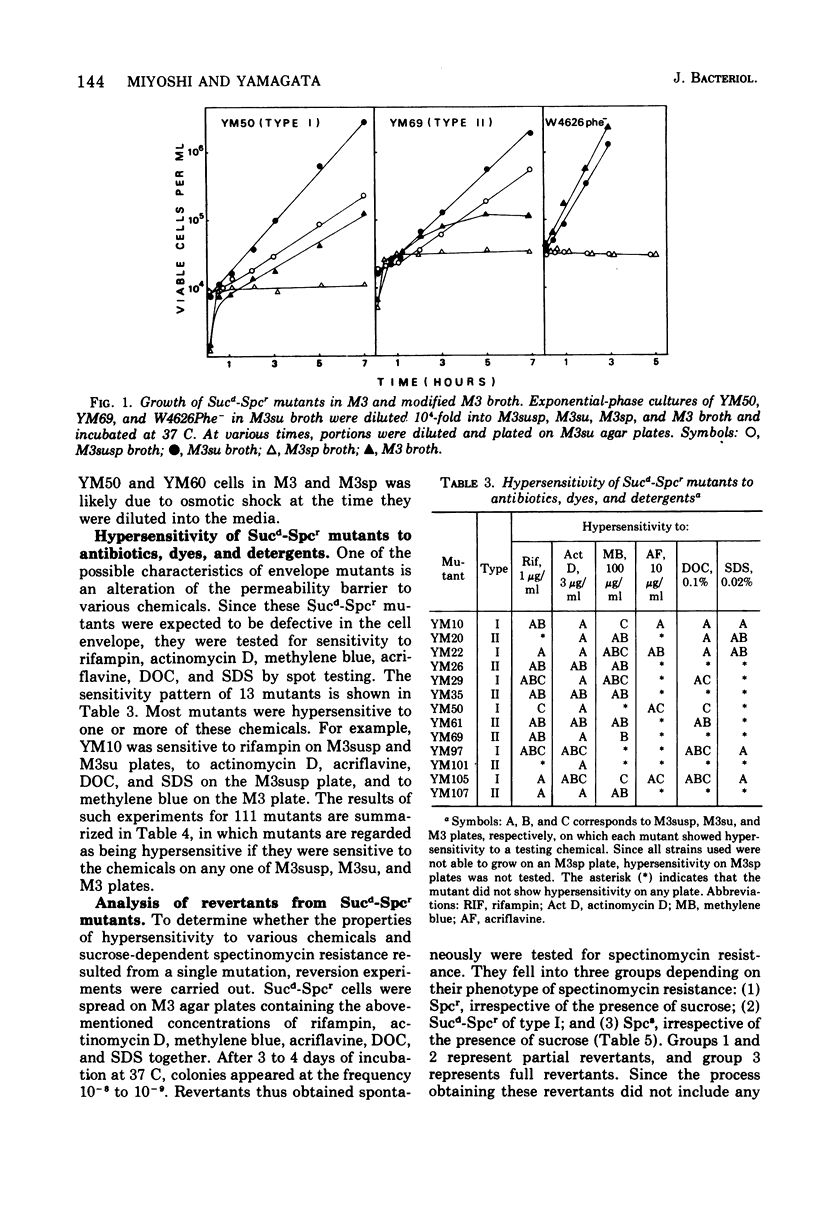
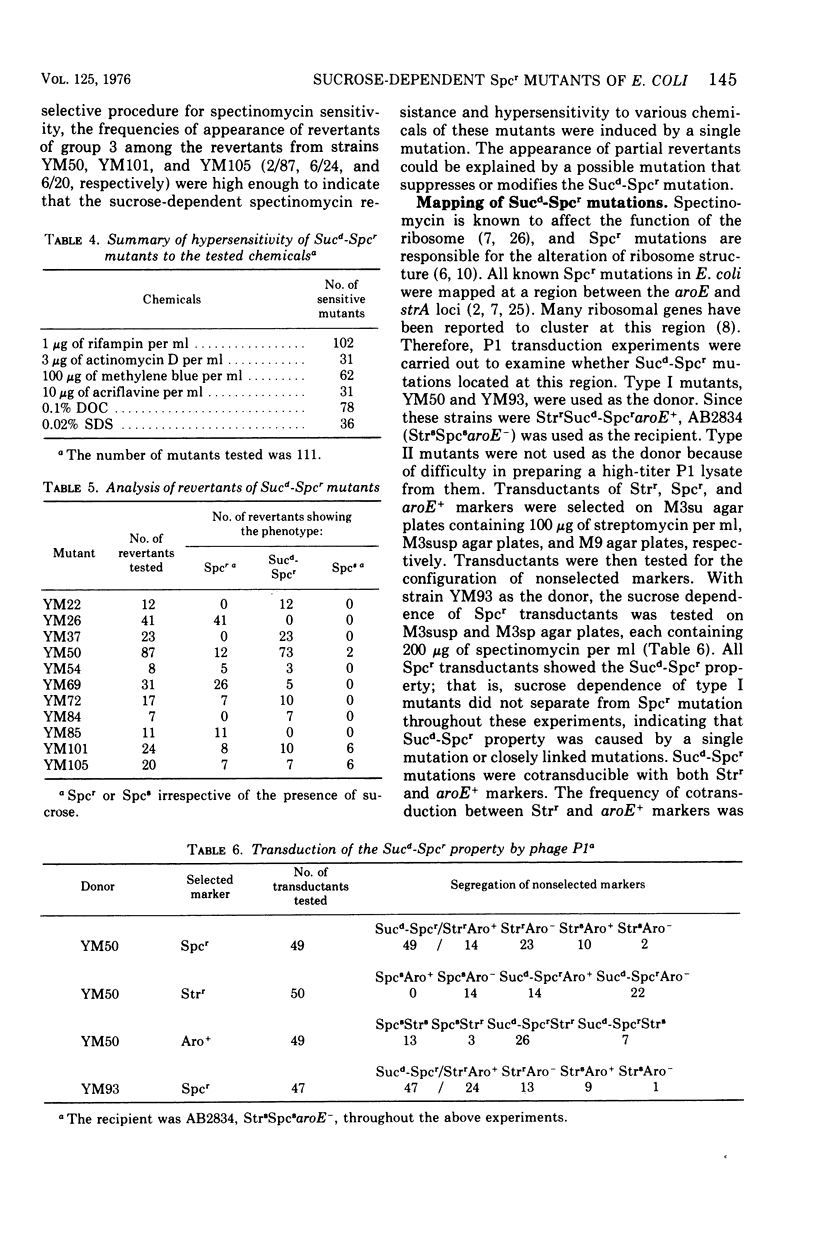
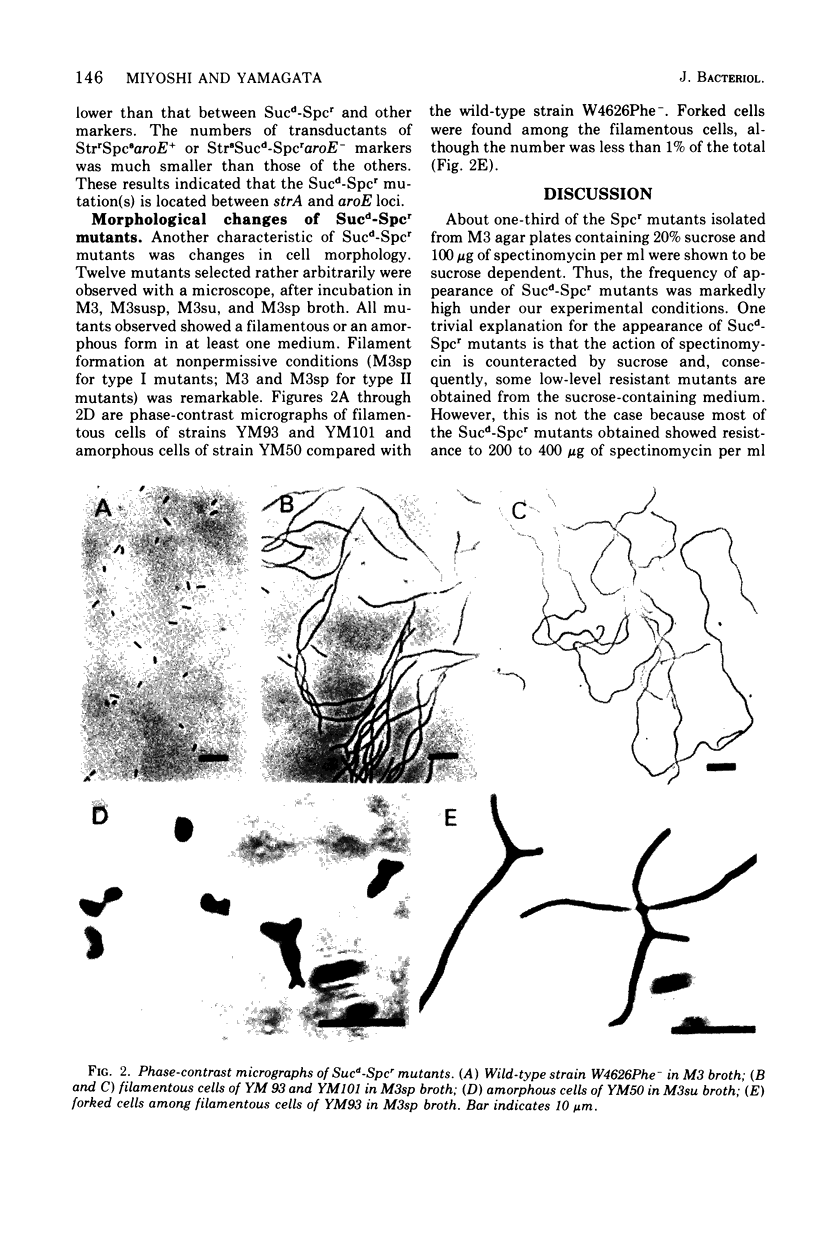
Spectinomycin-resistant (Spcr) mutants of Escherichia coli were isolated from nutrient agar plates containing 20% sucrose and 100 mug of spectinomycin per ml. About one-third of the Spcr mutants thus obtained were sucrose dependent (Sucd) and were classified into two types: I, those unable to grow on sucrose-free medium in the presence of spectinomycin; and II, those unable to grow on sucrose-free medium irrespective of the presence of spectinomycin. Most of these mutants were hypersensitive to antibiotics, dyes, and detergents and were abnormal in cell morphology, suggesting changes in cell envelopes. Reversion experiments indicated that the sucrose-dependent spectinomycin resistance and hypersensitivity to various chemicals were not independently induced properties. The Sucd-Spcr mutations of type I mutants were transducible by phage P1 and were mapped at the strA-aroE region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Davies J., Davis B. D. Effect of spectinomycin on polypeptide synthesis in extracts of Escherichia coli. J Mol Biol. 1967 Oct 14;29(1):203–215. doi: 10.1016/0022-2836(67)90191-x. [DOI] [PubMed] [Google Scholar]
- Anderson P. Sensitivity and Resistance to Spectinomycin in Escherichia coli. J Bacteriol. 1969 Nov;100(2):939–947. doi: 10.1128/jb.100.2.939-947.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón D. N. Osmotic-sensitive mutant of Salmonella typhimurium. J Bacteriol. 1972 Mar;109(3):1273–1283. doi: 10.1128/jb.109.3.1273-1283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky A. Z., Armstrong J. B. Osmotic reversal of temperature sensitivity in Escherichia coli. J Bacteriol. 1973 Jan;113(1):76–81. doi: 10.1128/jb.113.1.76-81.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Davies J., Ozaki M., Mizushima S. Ribosomal protein conferring sensitivity to the antibiotic spectinomycin in Escherichia coli. Science. 1968 Jul 4;165(3888):85–86. [PubMed] [Google Scholar]
- Bollen A., Helser T., Yamada T., Davies J. Altered ribosomes in antibiotic-resistant mutants of E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:95–100. doi: 10.1101/sqb.1969.034.01.015. [DOI] [PubMed] [Google Scholar]
- Brown M. E., Apirion D. Mapping a cluster of ribosomal genes in Escherichia coli. Mol Gen Genet. 1974;133(4):317–327. doi: 10.1007/BF00332707. [DOI] [PubMed] [Google Scholar]
- Davies J., Anderson P., Davis B. D. Inhibition of protein synthesis by spectinomycin. Science. 1965 Sep 3;149(3688):1096–1098. doi: 10.1126/science.149.3688.1096. [DOI] [PubMed] [Google Scholar]
- Dekio S., Takata R. Genetic studies of the ribosomal proteins in Escherichia. coli. II. Altered 30s ribosomal protein component specific to spectinomycin resistant mutants. Mol Gen Genet. 1969;105(3):219–224. doi: 10.1007/BF00337473. [DOI] [PubMed] [Google Scholar]
- Hendler R. W. Importance of membranes in protein biosynthesis. Nature. 1965 Sep 4;207(5001):1053–passim. doi: 10.1038/2071053a0. [DOI] [PubMed] [Google Scholar]
- Holland I. B., Threlfall E. J., Holland E. M., Darby V., Samson A. C. Mutants of Escherichia coli with altered surface properties which are refractory to colicin E2, sensitive to ultraviolet light and which can also show recombination deficiency, abortive growth of bacteriophage lambda and filament formation. J Gen Microbiol. 1970 Aug;62(3):371–382. doi: 10.1099/00221287-62-3-371. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Endo H., Ohnishi Y. Mutations to spectinomycin resistance which alleviate the restriction of an amber suppressor by streptomycin resistance. J Bacteriol. 1969 Feb;97(2):940–943. doi: 10.1128/jb.97.2.940-943.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D. Selection of sucrose-dependent Escherichia coli to obtain envelope mutants and fragile cultures. Science. 1966 Aug 19;153(3738):892–894. doi: 10.1126/science.153.3738.892. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968 Oct;96(4):987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashimoto H., Nomura M. Structure and function of bacterial ribosomes. XI. Dependence of 50S ribosomal assembly on simultaneous assembly of 30S subunits. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1440–1447. doi: 10.1073/pnas.67.3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siccardi A. G., Shapiro B. M. On the process of cellular division in Escherichia coli. IV. Altered protein composition and turnover of the membranes of thermosensitive mutants defective in chromosomal replication. J Mol Biol. 1971 Mar 28;56(3):475–490. doi: 10.1016/0022-2836(71)90395-0. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. J., Tai P. C., Davis B. D. Selective inhibition of initiating ribosomes by spectinomycin. Proc Natl Acad Sci U S A. 1974 May;71(5):1634–1638. doi: 10.1073/pnas.71.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Effect of acridine orange on sex factor multiplication in Escherichia coli. J Mol Biol. 1969 Nov 28;46(1):73–84. doi: 10.1016/0022-2836(69)90058-8. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]