Abstract
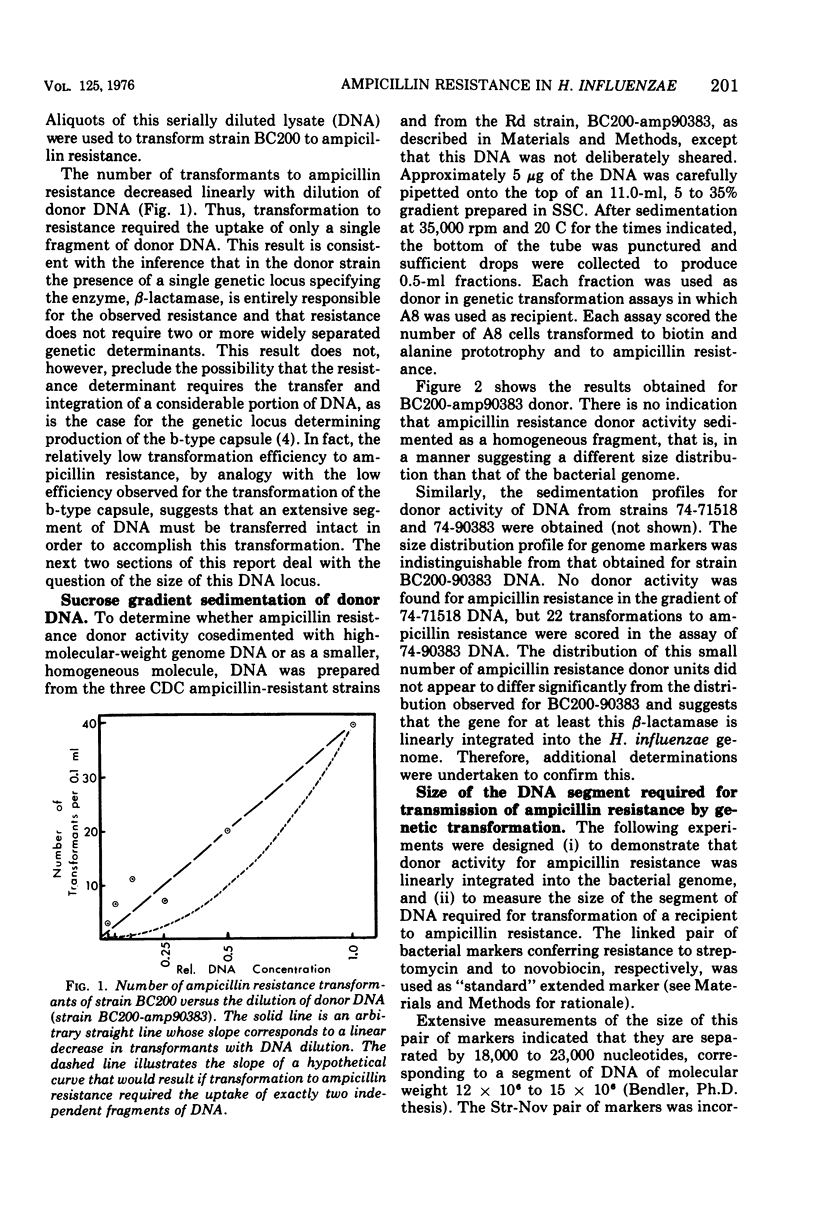
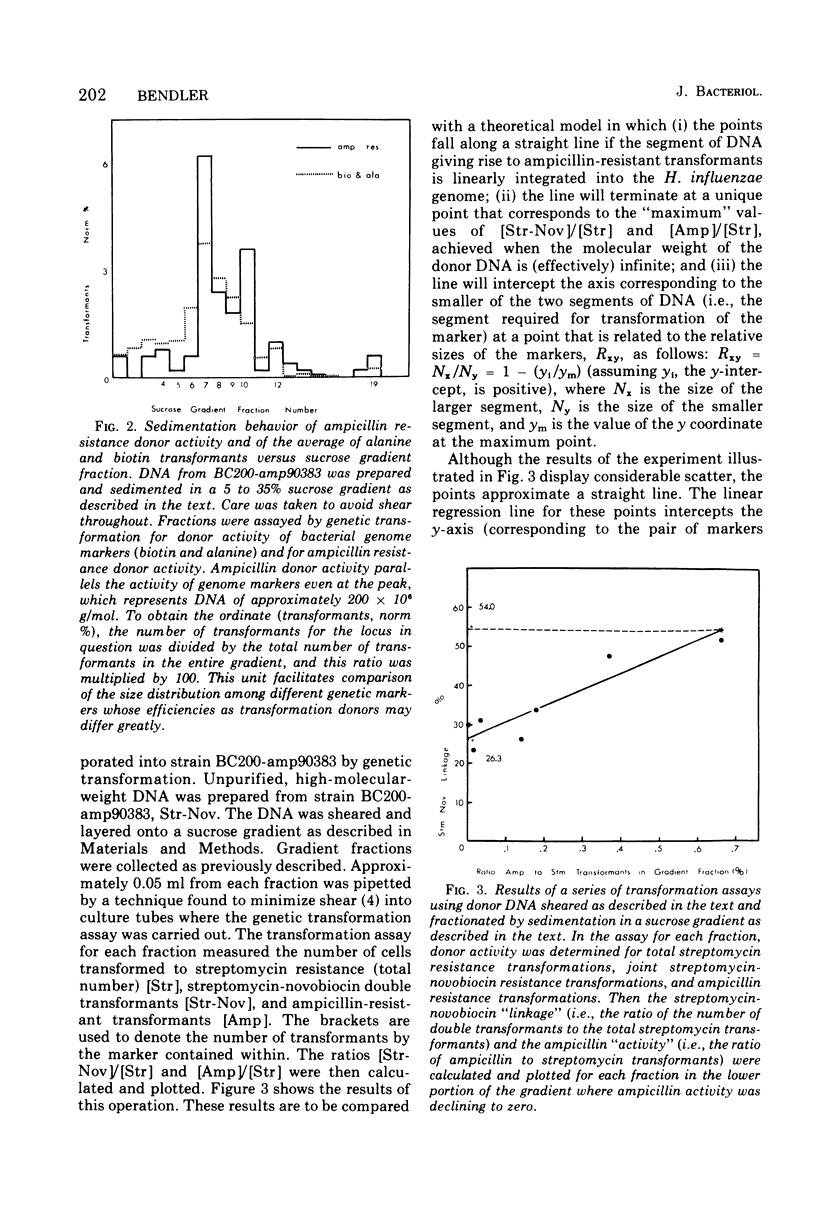
The properties of donor deoxyribonucleic acid (DNA) from three clinical isolates and its ability to mediate the transformation of competent Rd strains to ampicillin resistance were examined. A quantitative technique for determining the resistance of individual Haemophilus influenzae cells to ampicillin was developed. When this technique was used, sensitive cells failed to tolerate levels of ampicillin greater than 0.1 to 0.2 mug/ml, whereas three resistant type b beta-lactamase-producing strains could form from the colonies in 1- to 3-mug/ml levels of the antibiotic. DNA extracted from the resistant strains elicited transformation of the auxotrophic genes in a multiply auxotrophic Rd strain. For two of the donors, transformation to ampicillin resistance occurred after the uptake of a single DNA molecule approximately 104-fold less frequently than transformation of auxotrophic loci and was not observed to occur at all with the third. The frequency of transformation to ampicillin resistance was two- to fivefold higher in strain BC200 (Okinaka and Barnhart, 1974), which was cured of a defective prophage. All three clinical ampicillin-resistant strains were poor recipients, but the presence of the ampicillin resistant genes in strain BC200 did not reduce its competence. Sucrose gradients of DNA from ampicillin-resistant transformants of BC200 and from the original ampicillin-resistant strains showed that: (i) all the DNA preparations had high molecular weights; (ii) donor activity for ampicillin resistance sedimented heterogeneously and in parallel with genome DNA up to the highest molecular weights observed (100 x 106 to 200 x 106); and (iii) genetic transformation of ampicillin resistance from strain BC200-amp90383 required the physical integrity of a linearly integrated segment of DNA having a molecular weight of about 25 x 106 to 30 x 106.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Bendler J. W., Goodgal S. H. Prophage S2 mutants in Haemophilus influenzae: a technique for their production and isolation. Science. 1968 Oct 25;162(3852):464–465. doi: 10.1126/science.162.3852.464. [DOI] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Iodometric detection of Haemophilus influenzae beta-lactamase: rapid presumptive test for ampicillin resistance. Antimicrob Agents Chemother. 1975 Mar;7(3):265–270. doi: 10.1128/aac.7.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkova R., Bendler J., Goodgal S. Restriction and modification of bacteriophage S2 in Haemophilus influenzae. J Bacteriol. 1973 Jun;114(3):1151–1157. doi: 10.1128/jb.114.3.1151-1157.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Michalka J., Goodgal S. H. Genetic and physical map of the chromosome of Hemophilus influenzae. J Mol Biol. 1969 Oct 28;45(2):407–421. doi: 10.1016/0022-2836(69)90115-6. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Allison D. P. Intracellular events during infection by Haemophilus influenzae phage and transfection by its DNA. J Mol Biol. 1973 Apr 25;75(4):581–599. doi: 10.1016/0022-2836(73)90293-3. [DOI] [PubMed] [Google Scholar]
- Okinaka R. T., Barnhart B. J. Accumulation of incomplete particles after uv-light induction of Haemophilus influenzae lysogenic for bacteriophage HP1C1. J Virol. 1974 Dec;14(6):1604–1606. doi: 10.1128/jvi.14.6.1604-1606.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Samuels J., Clarke J. K. New bacteriophage of Haemophilus influenzae. J Virol. 1969 Nov;4(5):797–798. doi: 10.1128/jvi.4.5.797-798.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachura I., Mckinley F. W., Leidy G., Alexander H. E. Incomplete bacteriophage-like particles in ultraviolet-irradiated haemophilus. J Bacteriol. 1969 May;98(2):818–820. doi: 10.1128/jb.98.2.818-820.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne G. M., Farrar W. E., Jr Transfer of ampicillin resistance between strains of Haemophilus influenzae type B. J Infect Dis. 1975 Sep;132(3):276–281. doi: 10.1093/infdis/132.3.276. [DOI] [PubMed] [Google Scholar]



