Abstract
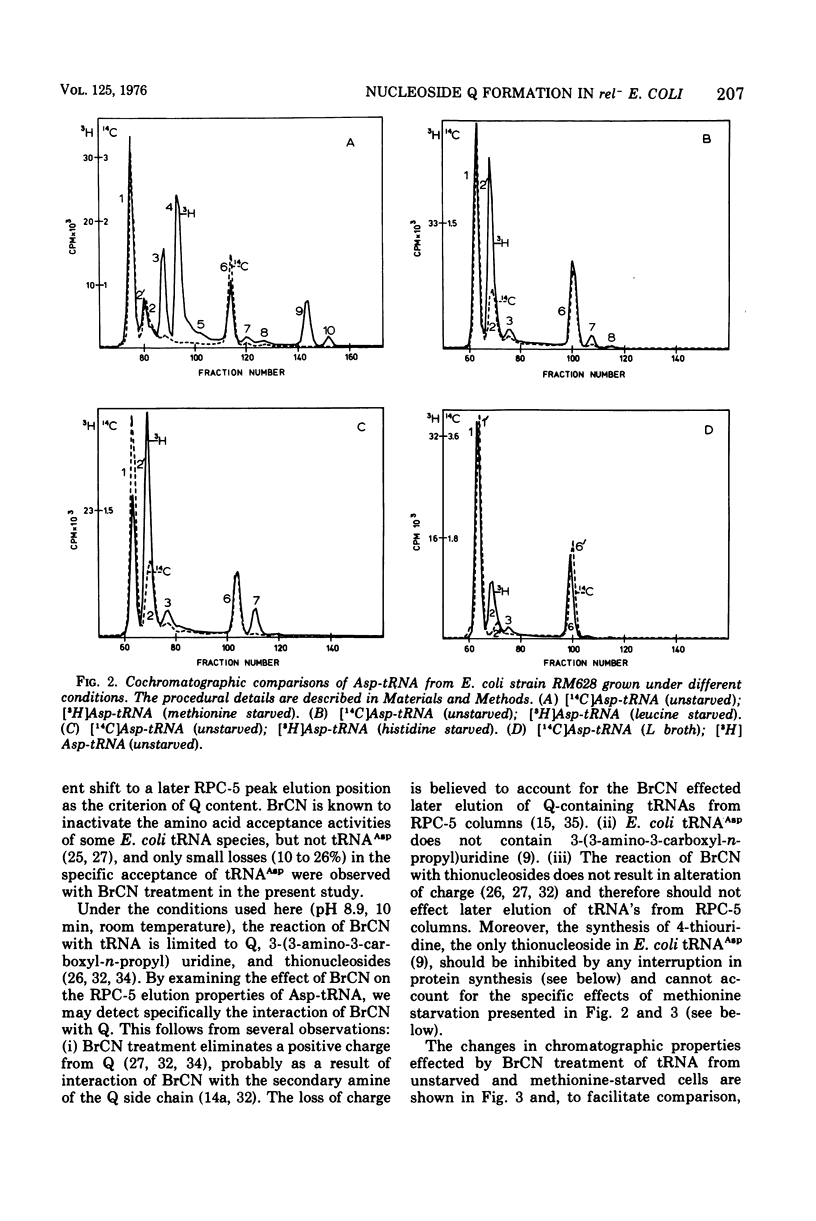
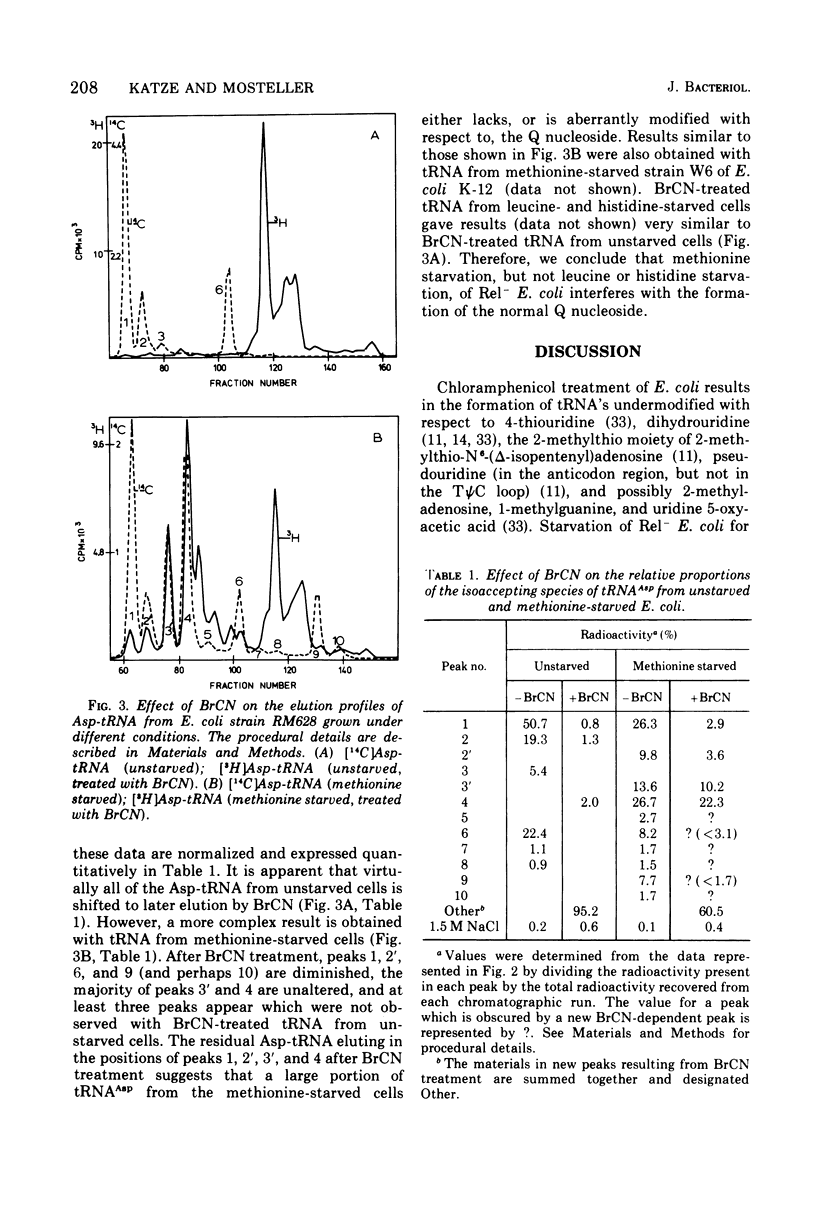
The elution profiles of Asp-tRNA from unstarved and starved cultures of a relaxed-control (Rel-) strain of Escherichia coli were compared by reversed-phase chromatography. Methionine starvation results in the appearance of several additional species of Asp-tRNA which are not observed with starvation for leucine or histidine. By the criterion of cyanogen bromide-effected shifts in chromatographic elution position, a large portion of the tRNAAsp synthesized in methionine-starved cells lacks the normal Q nucleoside. By the same criterion, virtually all of the tRNAAsp from unstarved, leucine-starved, and histidine-starved cells contain Q. We conclude that methionine starvation prevents the formation of the norma Q nucleoside in Rel- E. coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Armstrong D. J., Schäfer K. P., Söll D. Maturation of a hypermodified nucleoside in transfer RNA. Nucleic Acids Res. 1975 May;2(5):691–698. doi: 10.1093/nar/2.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J., Söll D., Burrows W. J., Skoog F. Identification of the cytokinin-active ribonucleosides in pure Escherichia coli tRNA species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1448–1453. doi: 10.1073/pnas.67.3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase R., Tener G. M., Gillam I. C. Changes in levels of amino acid acceptors in tRNA from Escherichia coli grown under various conditions. Arch Biochem Biophys. 1974 Jul;163(1):306–317. doi: 10.1016/0003-9861(74)90481-0. [DOI] [PubMed] [Google Scholar]
- FLEISSNER E., BOREK E. STUDIES ON THE ENZYMATIC METHYLATION OF SOLUBLE RNA. I. METHYLATION OF THE S-RNA POLYMER. Biochemistry. 1963 Sep-Oct;2:1093–1100. doi: 10.1021/bi00905a032. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Peterkofsky A. Formation of chromatographically unique species of transfer ribonucleic acid during amino acid starvation of relaxed-control Escherichia coli. J Bacteriol. 1975 May;122(2):538–548. doi: 10.1128/jb.122.2.538-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J. N., Landy A., Zadrazil S., Smith J. D. The nucleotide sequences of tyrosine transfer RNAs of Escherichia coli. Eur J Biochem. 1970 Apr;13(3):461–483. doi: 10.1111/j.1432-1033.1970.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Raab C. In vivo synthesis of tRNA Tyr 1 and tRNA Tyr 2 : differences in "early" and "late log" E. coli MRE 600. Biochem Biophys Res Commun. 1972 Mar 24;46(6):2006–2011. doi: 10.1016/0006-291x(72)90751-6. [DOI] [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
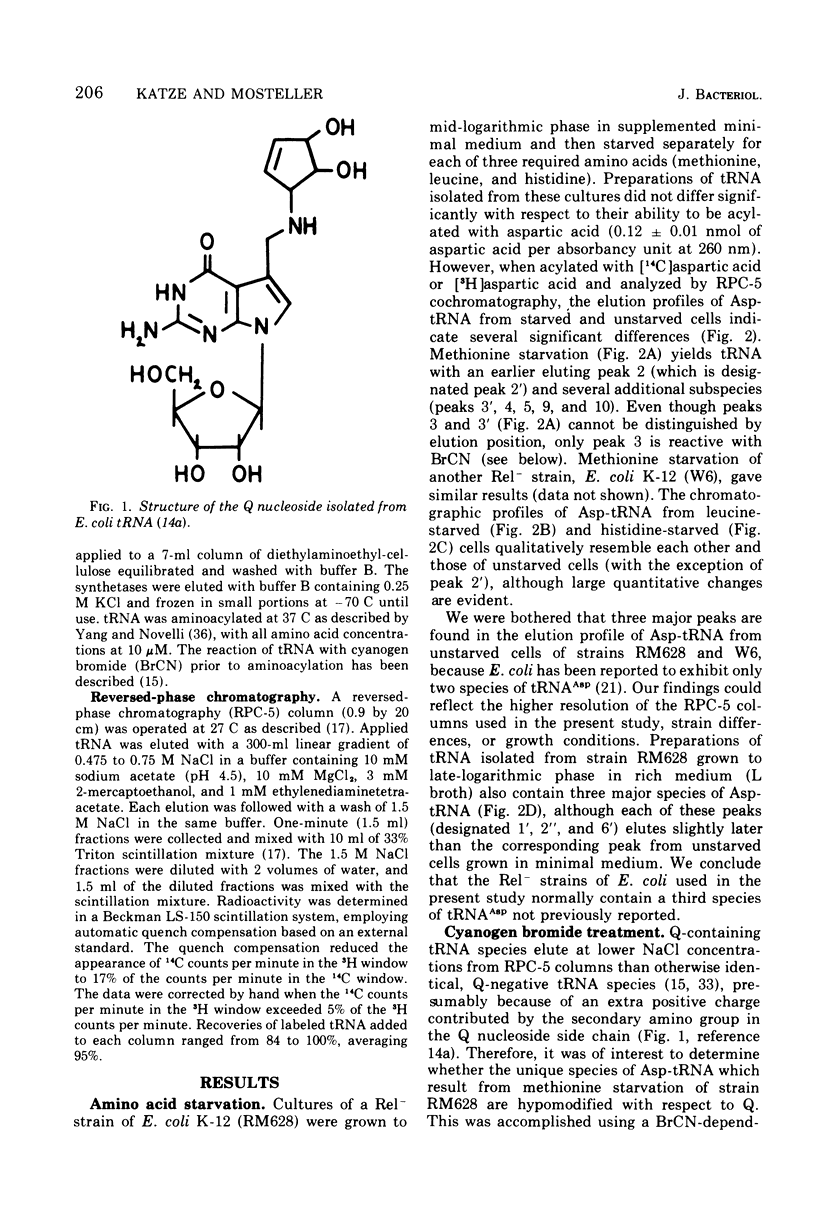
- Harada F., Yamaizumi K., Nishimura S. Oligonucleotide sequences of RNase T 1 and pancreatic RNase digests of E. coli aspartic acid tRNA. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1605–1609. doi: 10.1016/0006-291x(72)90525-6. [DOI] [PubMed] [Google Scholar]
- Harris C. L., Titchener E. B. Sulfur-deficient transfer ribonucleic acid. The natural substrate for ribonucleic acid sulfurtransferase from Escherichia coli. Biochemistry. 1971 Nov;10(23):4207–4212. doi: 10.1021/bi00799a008. [DOI] [PubMed] [Google Scholar]
- Huang P. C., Mann M. B. Comparative fingerprint and composition analysis of the three forms of 32P-labeled phenylalanine tRNA from chloramphenicol-treated Escherichia coli. Biochemistry. 1974 Nov 5;13(23):4704–4710. doi: 10.1021/bi00720a004. [DOI] [PubMed] [Google Scholar]
- Isham K. R., Stulberg M. P. Modified nucleosides in undermethylated phenylalanine transfer RNA from Escherichia coli. Biochim Biophys Acta. 1974 Mar 8;340(2):177–182. doi: 10.1016/0005-2787(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Hedgcoth C. Levels of 5,6-dihydrouridine in relaxed and chloramphenicol transfer ribonucleic acid. Biochemistry. 1970 Jun 9;9(12):2513–2519. doi: 10.1021/bi00814a018. [DOI] [PubMed] [Google Scholar]
- Juarez H., Skjold A. C., Hedgcoth C. Precursor relationship of phenylalanine transfer ribonucleic acid from Escherichia coli treated with chloramphenicol or starved for iron, methionine, or cysteine. J Bacteriol. 1975 Jan;121(1):44–54. doi: 10.1128/jb.121.1.44-54.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975 Sep 23;14(19):4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Katze J. R., Mason K. H. Comparison of the acceptance activity of the ribosome-bound and the total cellular transfer ribonucleic acids from SV40-transformed mouse fibroblasts. Biochim Biophys Acta. 1973 Dec 21;331(3):369–381. doi: 10.1016/0005-2787(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Fournier M. J. Inhibition of post-transcriptional modification of E. coli tRNA. Brookhaven Symp Biol. 1975 Jul;(26):44–52. [PubMed] [Google Scholar]
- Kivity-Vogel T., Elson D. On the metabolic inactivation of messenger RNA in Escherichia coli: ribonuclease I and polynucleotide phosphorylase. Biochim Biophys Acta. 1967 Mar 29;138(1):66–75. doi: 10.1016/0005-2787(67)90586-2. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Huang P. C. New chromatographic form of phenylalanine transfer ribonucleic acid from Escherichia coli growing exponentially in a low-phosphate medium. J Bacteriol. 1974 Apr;118(1):209–212. doi: 10.1128/jb.118.1.209-212.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench K. H., Safille P. A. Transfer ribonucleic acids in Escherichia coli. Multiplicity and variation. Biochemistry. 1968 Aug;7(8):2799–2808. doi: 10.1021/bi00848a015. [DOI] [PubMed] [Google Scholar]
- Munns T. W., Sims H. F. Methylation and processing of transfer ribonucleic acid in mammalian and bacterial cells. J Biol Chem. 1975 Mar 25;250(6):2143–2149. [PubMed] [Google Scholar]
- Münch H. J., Thiebe R. Biosynthesis of the nucleoside Y in yeast tRNAPhe: incorporation of the 3-amino-3-carboxypropyl-group from methionine. FEBS Lett. 1975 Mar 1;51(1):257–258. doi: 10.1016/0014-5793(75)80900-8. [DOI] [PubMed] [Google Scholar]
- Rao Y. S., Cherayil J. D. Studies on chemical modification of thionucleosides in the transfer ribonucleic acid of Escherichia coli. Biochem J. 1974 Nov;143(2):285–294. doi: 10.1042/bj1430285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi M., Nishimura S. Selective inactivation of amino acid acceptor and ribosome-binding activities of Escherichia coli tRNA by modification with cyanogen bromide. Biochim Biophys Acta. 1971 Aug 12;246(1):123–131. doi: 10.1016/0005-2787(71)90077-3. [DOI] [PubMed] [Google Scholar]
- Saneyoshi M., Nishimura S. Selective modification of 4-thiouridylate residue in Escherichia coli transfer RNA with cyanogen bromide. Biochim Biophys Acta. 1970 Apr 15;204(2):389–399. doi: 10.1016/0005-2787(70)90158-9. [DOI] [PubMed] [Google Scholar]
- Saponara A. G., Enger M. D. The isolation from ribonucleic acid of substituted uridines containing alpha-aminobutyrate moieties derived from methionine. Biochim Biophys Acta. 1974 Apr 27;349(1):61–77. doi: 10.1016/0005-2787(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Comer M. M., McClain W. H. Nucleotide alterations in the bacteriophage T4 glutamine transfer RNA that affect ochre suppressor activity. J Mol Biol. 1974 Dec 25;90(4):677–689. doi: 10.1016/0022-2836(74)90532-4. [DOI] [PubMed] [Google Scholar]
- Singhal R. P., Best A. N. Examination of highly purified transfer RNAs from Escherichia coli. Differences in amount of minor components and presence of a cytidine-thiouridine photoproduct in "normal" tRNAs; a comparison of two analytical methods. Biochim Biophys Acta. 1973 Dec 21;331(3):357–368. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Studies on polynucleotides. CI. Escherichia coli tyrosine and formylmethionine transfer ribonucleic acids: effect of chemical modification of 4-thiouridine to uridine on their biological properties. J Biol Chem. 1972 Aug 10;247(15):4879–4892. [PubMed] [Google Scholar]
- Waters L. C., Shugart L., Yang W. K., Best A. N. Some physical and biological properties of 4-thiouridine- and dihydrouridine-deficient tRNA from chloramphenicol-treated Escherichia coli. Arch Biochem Biophys. 1973 Jun;156(2):780–793. doi: 10.1016/0003-9861(73)90332-9. [DOI] [PubMed] [Google Scholar]
- White B. N. Chromatographic changes in specific tRNAs after reaction with cyanogen bromide and sodium periodate. Biochim Biophys Acta. 1974 Jul 11;353(3):283–291. doi: 10.1016/0005-2787(74)90021-5. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]



