Abstract
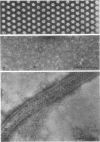
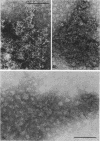
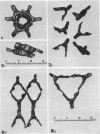
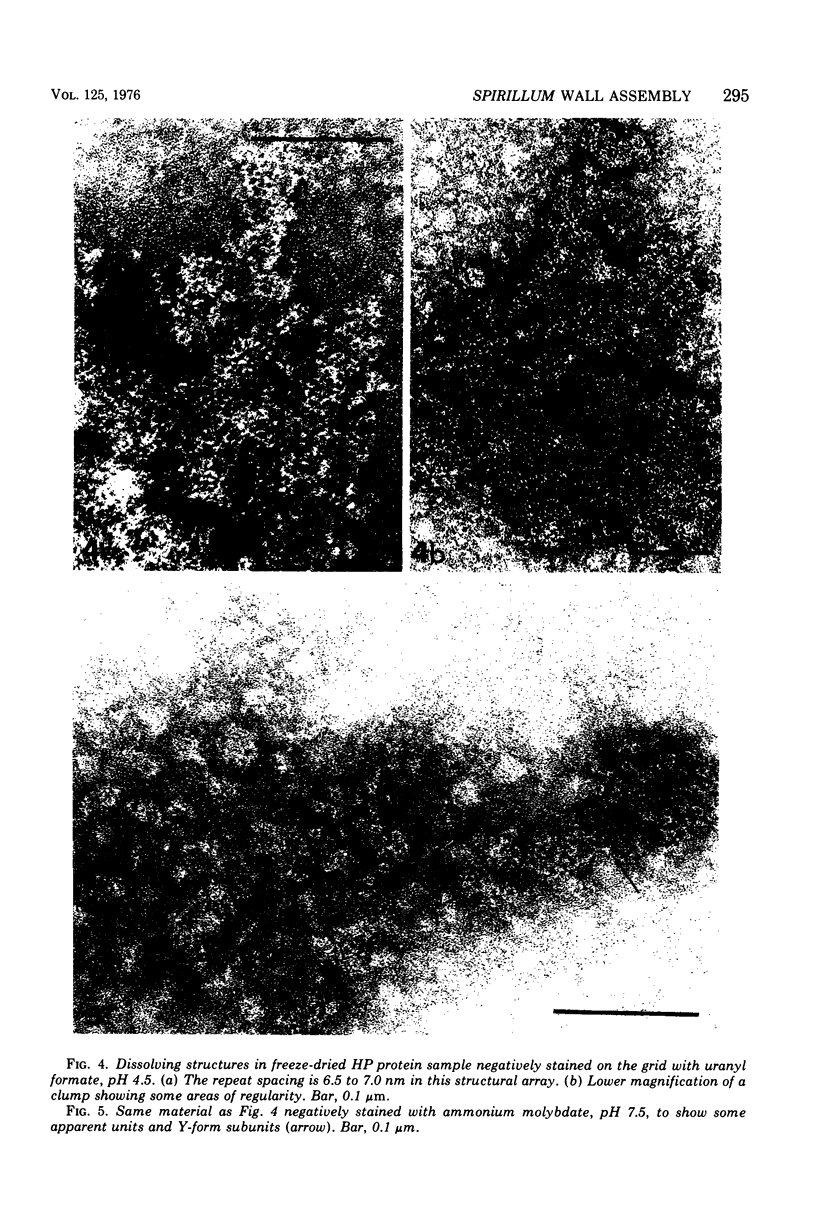
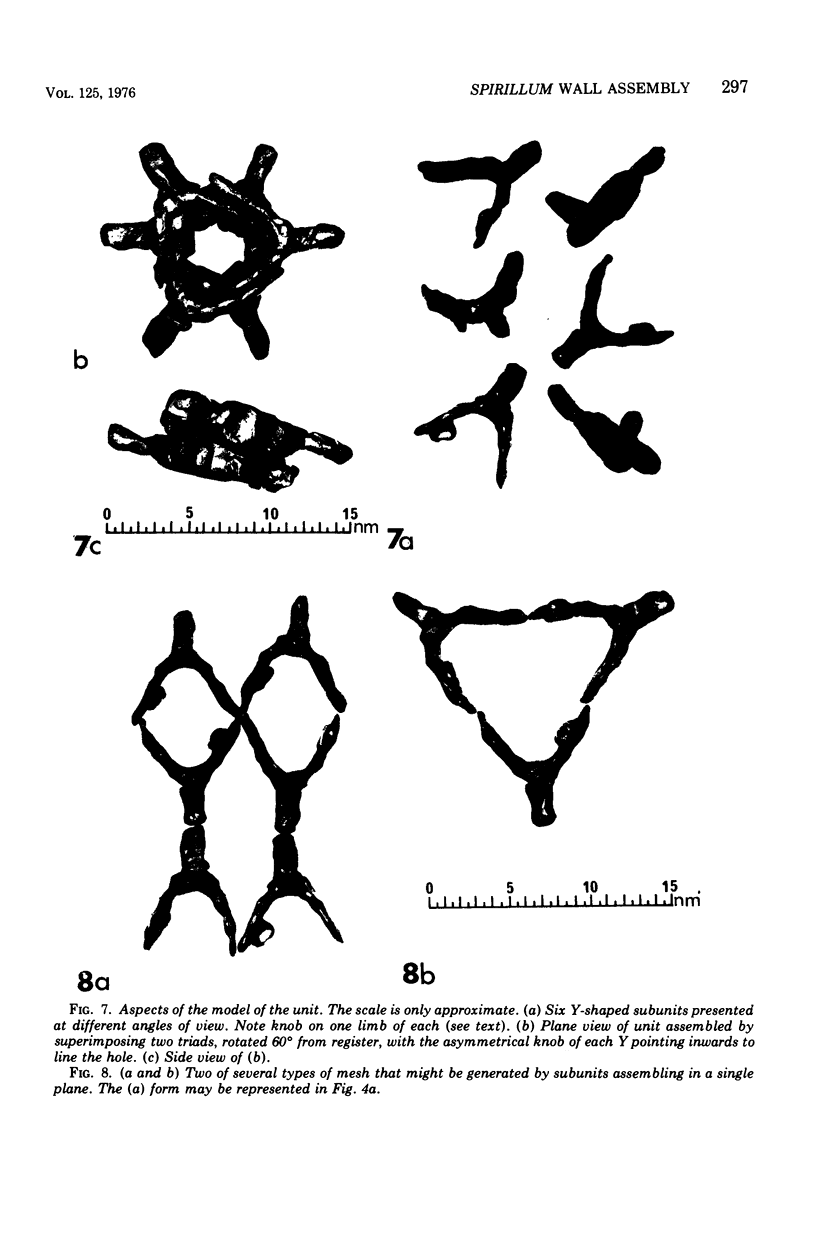
Electron micrographs of disintegrating units of the outer, structured (HP) layer of Spirillum serpens and of the isolated protein obtained from the HP layer revealed V- and Y-shaped and linear profiles. Interpretation of these forms, influenced by the seemingly trimeric form of the isolated protein and by biochemical data, suggested that the protein subunits were identical and Y shaped. A model is proposed for the assembly of the Y-shaped subunits to form a hexagon composed of two triads (three Y-shaped subunits each). The isolated protein adsorbed to a template of wall fragments (basal layer) to the same degree (over 90%) in high concentrations of Na+, K+ (5 X 10(-2) M), Ca2+, Sr2+, and Mg2+ (10(-2) M). At a lower concentration (4 x 10(-5) M) of the cations there was differential adsorption of the protein. Adsorption to the template in the presence of each cation, followed by dilution, also led to differential release of the protein. The adsorption of the protein to the basal layer was correlated with reassembly of the HP layer on the template. The mechanisms seem to be: (i) an ionic strength-dependent reassembly, which results in an HP layer loosely attached to the template (this layer is easily dissociated by decreasing the ionic strength); and (ii) a cation-specific (Ca2+ or Sr2+, but not Mg2+, Na+, or K+) mechanism independent of ionic strength. In this latter case, the specific cations presumably form strong noncovalent "salt" linkages between triads and the basal layer, enabling stable hexagons and the HP layer to be formed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- Howard L., Tipper D. J. A polypeptide bacteriophage receptor: modified cell wall protein subunits in bacteriophage-resistant mutants of Bacillus sphaericus strain P-1. J Bacteriol. 1973 Mar;113(3):1491–1504. doi: 10.1128/jb.113.3.1491-1504.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKHAM R., HITCHBORN J. H., HILLS G. J., FREY S. THE ANATOMY OF THE TOBACCO MOSAIC VIRUS. Virology. 1964 Mar;22:342–359. doi: 10.1016/0042-6822(64)90025-x. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Thorne K. J., Glauert A. M. Detachment and chemical characterization of the regularly arranged subunits from the surface of an Acinetobacter. J Bacteriol. 1974 May;118(2):654–662. doi: 10.1128/jb.118.2.654-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]