Abstract
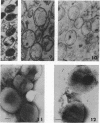
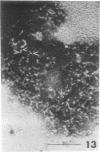
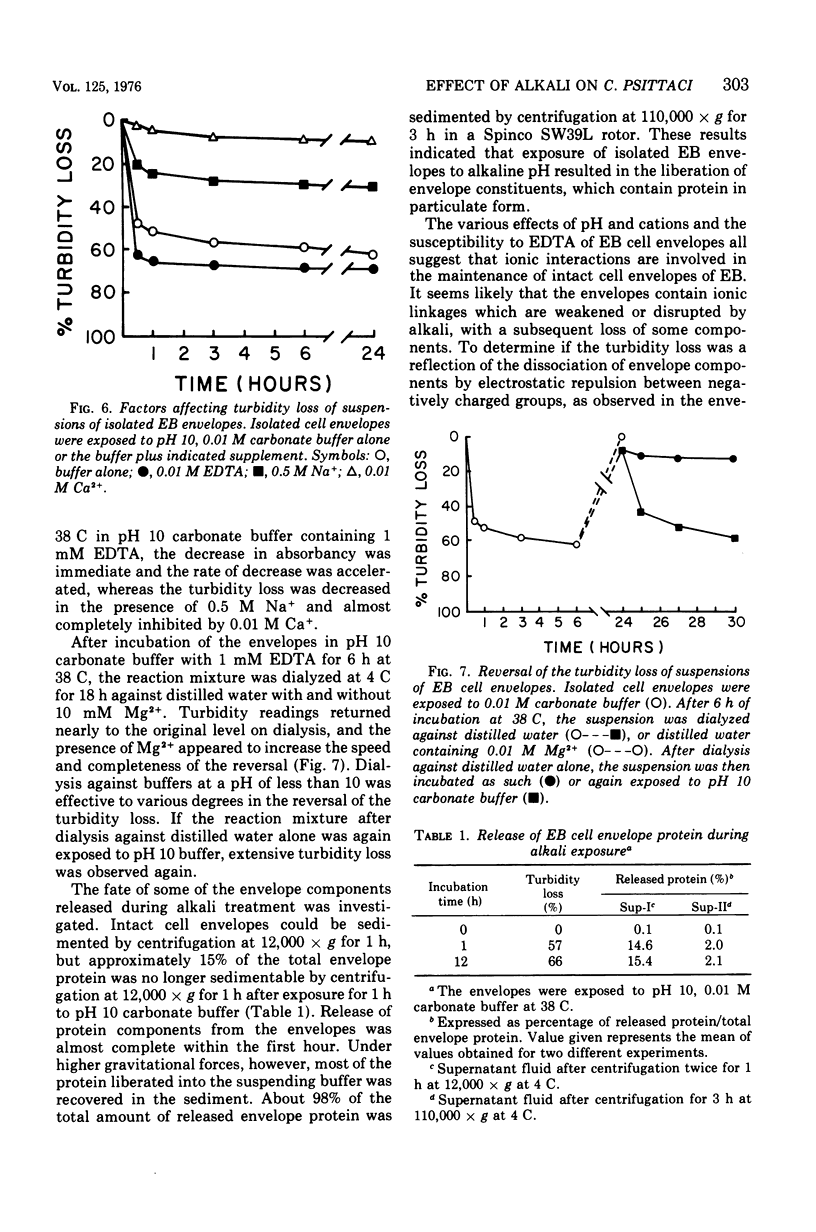
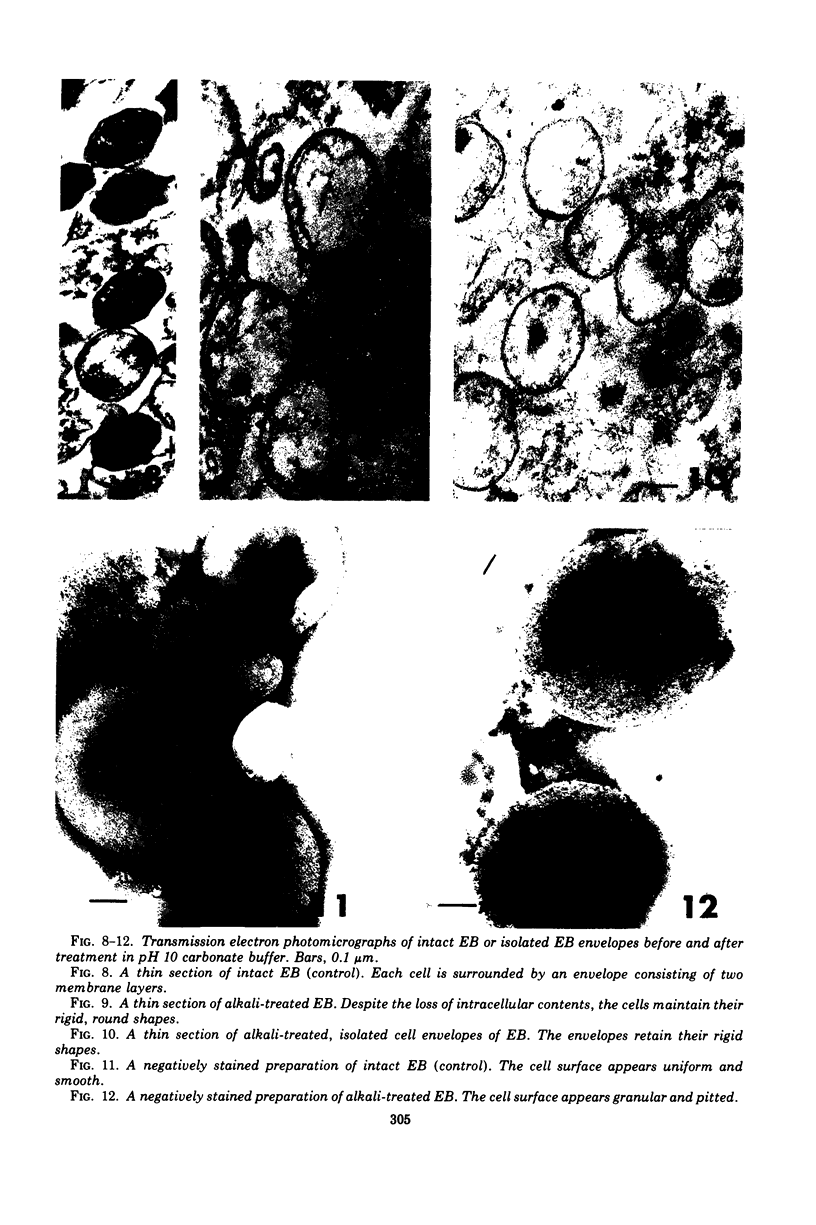
Suspensions of isolated cell envelopes of infectious elementary bodies (EB) of Chlamydia psittaci at alkaline pH showed a rapid, extensive decrease in absorbance, accompanied by the release of a cell envelope component in a sedimentable form. This phenomenon was observed both at 0 C and with envelopes which had been previously heated to 100 C. Monovalent and divalent cations effectively inhibited the turbidity loss, whereas ethylenediaminetetraacetate (EDTA) caused an accelerated decrease in turbidity. The turbidity loss observed after incubation of the envelopes at alkaline pH could be reversed to the level of the initial value by dialysis against distilled water containing Mg2+. Thin-section electron photomicrographs of purified EB exposed to alkaline buffer with EDTA revealed the loss of the internal contents of cells, but these cells still maintained their round shapes. The cell surface of treated EB appeared pitted in negatively stained preparations, whereas intact EB had a smooth surface. Electron microscopic studies on negatively stained preparations of the clear supernatant obtained after the treatment of the envelope with alkaline buffer containing EDTA demonstrated the presence of spherical particles, approximately 6 to 7 nm in diameter, and rodlike particles, which appeared to be made up of two or more spherical particles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A. D. ASPECTS OF BACTERIAL RESPONSE TO THE IONIC ENVIRONMENT. Bacteriol Rev. 1964 Sep;28:296–329. doi: 10.1128/br.28.3.296-329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN A. D. THE DEVELOPMENT OF HALOPHILIC PROPERTIES IN BACTERIAL MEMBRANES BY ACYLATION. Biochim Biophys Acta. 1964 Oct 9;93:136–142. doi: 10.1016/0304-4165(64)90267-3. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XIV. On the mechanism of lysis of a marine bacterium. Can J Microbiol. 1965 Aug;11(4):677–691. doi: 10.1139/m65-091. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Dissociation and reassembly of Escherichia coli outer membrane and of lipopolysaccharide, and their reassembly onto flagellar basal bodies. J Bacteriol. 1971 Mar;105(3):1184–1199. doi: 10.1128/jb.105.3.1184-1199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Cell wall binding properties of the Bacillus subtilis autolysin(s). J Bacteriol. 1970 Aug;103(2):488–493. doi: 10.1128/jb.103.2.488-493.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A. J., Harrison M. J., Manire G. P. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol. 1974 Jan;80(1):315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Roth I. L., Eagon R. G. Freeze-etch study of Pseudomonas aeruginosa: localization within the cell wall of an ethylenediaminetetraacetate-extractable. J Bacteriol. 1973 Jan;113(1):417–432. doi: 10.1128/jb.113.1.417-432.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Manire G. P., Tamura A. Preparation and chemical composition of the cell walls of mature infectious dense forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Higashi N., Tamura A. Electron microscope observations on the effects of polymixin B sulfate on cell walls of Chlamydia psittaci. J Bacteriol. 1973 Jan;113(1):357–364. doi: 10.1128/jb.113.1.357-364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron Microscopic Observations on the Fine Structure of Cell Walls of Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1332–1337. doi: 10.1128/jb.104.3.1332-1337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Langworth T. A., Mayberry W. R., Houghland A. E. Characterization of the membranes of Thermoplasma acidophilum. J Bacteriol. 1973 Nov;116(2):1019–1028. doi: 10.1128/jb.116.2.1019-1028.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Cytochrome C reductase activity of meningopneumonitis organisms at different stages of development. Proc Soc Exp Biol Med. 1968 Nov;129(2):390–393. doi: 10.3181/00379727-129-33328. [DOI] [PubMed] [Google Scholar]
- Tamura A., Manire G. P. Preparation and chemical composition of the cell membranes of developmental reticulate forms of meningopneumonitis organisms. J Bacteriol. 1967 Oct;94(4):1184–1188. doi: 10.1128/jb.94.4.1184-1188.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Manire G. P., Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and developmental reticulate forms of Chlamydia psittaci. J Bacteriol. 1971 Jan;105(1):355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]